The C-Reactive Protein to Albumin Ratio as a Predictor of Severe Side Effects of Adjuvant Chemotherapy in Stage III Colorectal Cancer Patients
- PMID: 27930703
- PMCID: PMC5145220
- DOI: 10.1371/journal.pone.0167967
The C-Reactive Protein to Albumin Ratio as a Predictor of Severe Side Effects of Adjuvant Chemotherapy in Stage III Colorectal Cancer Patients
Abstract
Background/aims: Adjuvant chemotherapy (AC) has been reported to improve the prognosis for patients with Stage III colorectal cancer (CRC). However, some patients experience severe side effects and must stop AC. The C-reactive protein (CRP) to albumin ratio (CAR) is a novel inflammation-based score that could reflect the patient's general condition. The aim of this study was to evaluate the predictive value of the CAR for side effects of AC in CRC.
Methods: A total of 136 CRC patients who received AC were retrospectively analyzed. The patients were subdivided into two groups by the CAR level (CAR ≥0.1, n = 30; CD < 0.1, n = 106).
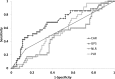
Results: The presence of lymphatic invasion, severe side effects, and discontinuation of AC were associated with high CAR levels (p = 0.02, <0.01, and 0.02; respectively). High levels of the Glasgow Prognostic Score (GPS) and the neutrophil to lymphocyte ratio (NLR) appeared to be associated with the CAR (p = 0.04, p<0.01; respectively). Multivariate analysis identified CAR≥0.1 (HR: 7.06, 95% CI: 2.51-19.88, p<0.01) as a significant determinant of severe side effects of AC. CAR had the highest area under the curve (0.79) among several inflammation-based scores.
Conclusion: The present study showed that the CAR is a novel and promising inflammation-based score for ≥ grade 3 side effects of AC in node-positive CRC.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG. AJCC cancer staging manual. 6th ed. Philadelphia: Springer; 2002
-
- Japanese Society for Cancer of the Colon and Rectum: multi-institutional registry of large bowel cancer in Japan. Vol. 30 cases treated in 2003–2004. Published in October 2012.
-
- NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990; 264: 1444–1450. - PubMed
-
- Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Multicentre international study of Oxaliplatin/5-Fluorouracil/Leucovorin in the adjuvant treatment of colon cancer (MOSAIC) Investigators: Oxaliplatin, fluorouracil, and leucovorin as surgical adjuvant treatment of colon cancer. N Engl J Med. 2004;350: 2343–2351. 10.1056/NEJMoa032709 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

