AP39, a mitochondria-targeting hydrogen sulfide (H2 S) donor, protects against myocardial reperfusion injury independently of salvage kinase signalling
- PMID: 27930802
- PMCID: PMC5289944
- DOI: 10.1111/bph.13688
AP39, a mitochondria-targeting hydrogen sulfide (H2 S) donor, protects against myocardial reperfusion injury independently of salvage kinase signalling
Abstract
Background and purpose: H2 S protects myocardium against ischaemia/reperfusion injury. This protection may involve the cytosolic reperfusion injury salvage kinase (RISK) pathway, but direct effects on mitochondrial function are possible. Here, we investigated the potential cardioprotective effect of a mitochondria-specific H2 S donor, AP39, at reperfusion against ischaemia/reperfusion injury.
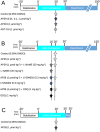
Experimental approach: Anaesthetized rats underwent myocardial ischaemia (30 min)/reperfusion (120 min) with randomization to receive interventions before reperfusion: vehicle, AP39 (0.01, 0.1, 1 μmol·kg-1 ), or control compounds AP219 and ADT-OH (1 μmol·kg-1 ). LY294002, L-NAME or ODQ were used to investigate the involvement of the RISK pathway. Myocardial samples harvested 5 min after reperfusion were analysed for RISK protein phosphorylation and isolated cardiac mitochondria were used to examine the direct mitochondrial effects of AP39.
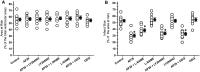
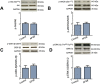
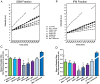
Key results: AP39, dose-dependently, reduced infarct size. Inhibition of either PI3K/Akt, eNOS or sGC did not affect this effect of AP39. Western blot analysis confirmed that AP39 did not induce phosphorylation of Akt, eNOS, GSK-3β or ERK1/2. In isolated subsarcolemmal and interfibrillar mitochondria, AP39 significantly attenuated mitochondrial ROS generation without affecting respiratory complexes I or II. Furthermore, AP39 inhibited mitochondrial permeability transition pore (PTP) opening and co-incubation of mitochondria with AP39 and cyclosporine A induced an additive inhibitory effect on the PTP.
Conclusion and implications: AP39 protects against reperfusion injury independently of the cytosolic RISK pathway. This cardioprotective effect could be mediated by inhibiting PTP via a cyclophilin D-independent mechanism. Thus, selective delivery of H2 S to mitochondria may be therapeutically applicable for employing the cardioprotective utility of H2 S.
© 2016 The British Pharmacological Society.
Figures



References
-
- Andreadou I, Iliodromitis EK, Rassaf T, Schulz R, Papapetropoulos A, Ferdinandy P (2015). The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br J Pharmacol 172: 1587–1606. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

