Increased mitochondrial ROS generation mediates the loss of the anti-contractile effects of perivascular adipose tissue in high-fat diet obese mice
- PMID: 27930804
- PMCID: PMC5610168
- DOI: 10.1111/bph.13687
Increased mitochondrial ROS generation mediates the loss of the anti-contractile effects of perivascular adipose tissue in high-fat diet obese mice
Abstract
Background and purpose: Obesity is associated with structural and functional changes in perivascular adipose tissue (PVAT), favouring release of reactive oxygen species (ROS), vasoconstrictor and proinflammatory factors. The cytokine TNF-α induces vascular dysfunction and is produced by PVAT. We tested the hypothesis that obesity-associated PVAT dysfunction was mediated by augmented mitochondrial ROS (mROS) generation due to increased TNF-α production in this tissue.
Experimental approach: C57Bl/6J and TNF-α receptor-deficient mice received control or high fat diet (HFD) for 18 weeks. We used pharmacological tools to determine the participation of mROS in PVAT dysfunction. Superoxide anion (O2.- ) and H2 O2 were assayed in PVAT and aortic rings were used to assess vascular function.
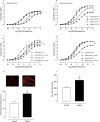
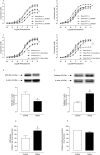
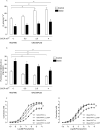
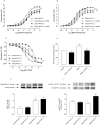
Key results: Aortae from HFD-fed obese mice displayed increased contractions to phenylephrine and loss of PVAT anti-contractile effect. Inactivation of O2.- , dismutation of mitochondria-derived H2 O2 , uncoupling of oxidative phosphorylation and Rho kinase inhibition, decreased phenylephrine-induced contractions in aortae with PVAT from HFD-fed mice. O2.- and H2 O2 were increased in PVAT from HFD-fed mice. Mitochondrial respiration analysis revealed decreased O2 consumption rates in PVAT from HFD-fed mice. TNF-α inhibition reduced H2 O2 levels in PVAT from HFD-fed mice. PVAT dysfunction, i.e. increased contraction to phenylephrine in PVAT-intact aortae, was not observed in HFD-obese mice lacking TNF-α receptors. Generation of H2 O2 was prevented in PVAT from TNF-α receptor deficient obese mice.
Conclusion and implications: TNF-α-induced mitochondrial oxidative stress is a key and novel mechanism involved in obesity-associated PVAT dysfunction. These findings elucidate molecular mechanisms whereby oxidative stress in PVAT could affect vascular function.
Linked articles: This article is part of a themed section on Molecular Mechanisms Regulating Perivascular Adipose Tissue - Potential Pharmacological Targets? To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.20/issuetoc.
© 2016 The British Pharmacological Society.
Figures
Similar articles
-
Restoration of perivascular adipose tissue function in diet-induced obese mice without changing bodyweight.Br J Pharmacol. 2017 Oct;174(20):3443-3453. doi: 10.1111/bph.13703. Epub 2017 Jan 31. Br J Pharmacol. 2017. PMID: 28055105 Free PMC article.
-
The role of perivascular adipose tissue in obesity-induced vascular dysfunction.Br J Pharmacol. 2017 Oct;174(20):3425-3442. doi: 10.1111/bph.13650. Epub 2016 Nov 17. Br J Pharmacol. 2017. PMID: 27761903 Free PMC article. Review.
-
Deletion of AMPKα1 attenuates the anticontractile effect of perivascular adipose tissue (PVAT) and reduces adiponectin release.Br J Pharmacol. 2017 Oct;174(20):3398-3410. doi: 10.1111/bph.13633. Epub 2016 Oct 23. Br J Pharmacol. 2017. PMID: 27668984 Free PMC article.
-
Endothelin-1 down-regulates nuclear factor erythroid 2-related factor-2 and contributes to perivascular adipose tissue dysfunction in obesity.Clin Sci (Lond). 2024 Sep 4;138(17):1071-1087. doi: 10.1042/CS20240624. Clin Sci (Lond). 2024. PMID: 39136472
-
Pro-contractile effects of perivascular fat in health and disease.Br J Pharmacol. 2017 Oct;174(20):3482-3495. doi: 10.1111/bph.13767. Epub 2017 Apr 3. Br J Pharmacol. 2017. PMID: 28257140 Free PMC article. Review.
Cited by
-
Perivascular Adipose Tissue as a Target for Antioxidant Therapy for Cardiovascular Complications.Antioxidants (Basel). 2020 Jul 2;9(7):574. doi: 10.3390/antiox9070574. Antioxidants (Basel). 2020. PMID: 32630640 Free PMC article. Review.
-
Male and female high-fat diet-fed Dahl SS rats are largely protected from vascular dysfunctions: PVAT contributions reveal sex differences.Am J Physiol Heart Circ Physiol. 2021 Jul 1;321(1):H15-H28. doi: 10.1152/ajpheart.00131.2021. Epub 2021 Apr 30. Am J Physiol Heart Circ Physiol. 2021. PMID: 33929898 Free PMC article.
-
Inhibition of IL-6 signaling prevents serum-induced umbilical cord artery dysfunction from patients with severe COVID-19.Am J Physiol Regul Integr Comp Physiol. 2023 Apr 1;324(4):R435-R445. doi: 10.1152/ajpregu.00154.2022. Epub 2023 Feb 3. Am J Physiol Regul Integr Comp Physiol. 2023. PMID: 36737252 Free PMC article.
-
The Local Regulation of Vascular Function: From an Inside-Outside to an Outside-Inside Model.Front Physiol. 2019 Jun 12;10:729. doi: 10.3389/fphys.2019.00729. eCollection 2019. Front Physiol. 2019. PMID: 31244683 Free PMC article. Review.
-
Suppressed vascular Rho-kinase activation is a protective cardiovascular mechanism in obese female mice.Biosci Rep. 2023 Jul 26;43(7):BSR20230672. doi: 10.1042/BSR20230672. Biosci Rep. 2023. PMID: 37342890 Free PMC article.
References
-
- Agabiti‐Rosei C, De Ciuceis C, Rossini C, Porteri E, Rodella LF, Withers SB et al. (2014). Anti‐contractile activity of perivascular fat in obese mice and the effect of long‐term treatment with melatonin. J Hypertens 32: 1264–1274. - PubMed
-
- Anusree SS, Nisha VM, Priyanka A, Raghu KG (2015). Insulin resistance by TNF‐α is associated with mitochondrial dysfunction in 3 T3‐L1 adipocytes and is ameliorated by punicic acid, a PPARγ agonist. Mol Cell Endocrinol 413: 120–128. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

