The impact of advertising patient and public involvement on trial recruitment: embedded cluster randomised recruitment trial
- PMID: 27931252
- PMCID: PMC5146878
- DOI: 10.1186/s13063-016-1718-1
The impact of advertising patient and public involvement on trial recruitment: embedded cluster randomised recruitment trial
Abstract
Background: Patient and public involvement in research (PPIR) may improve trial recruitment rates, but it is unclear how. Where trials use PPIR to improve design and conduct, many do not communicate this clearly to potential participants. Better communication of PPIR might encourage patient enrolment, as trials may be perceived as more socially valid, relevant and trustworthy. We aimed to evaluate the impact on recruitment of directly advertising PPIR to potential trial participants.
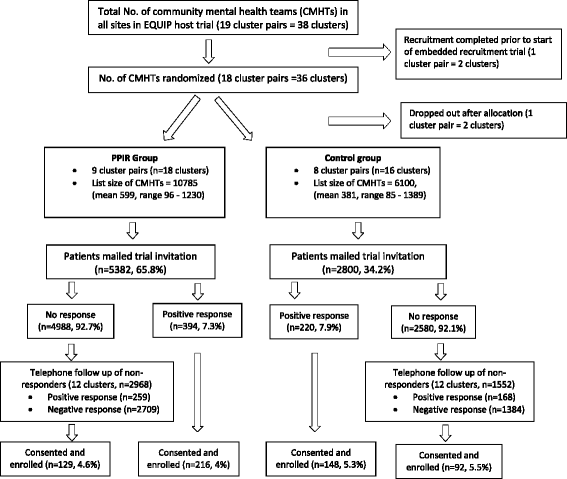
Methods: This is a cluster trial, embedded within a host trial ('EQUIP') recruiting service users diagnosed with severe mental illness. The intervention was informed by a systematic review, a qualitative study, social comparison theory and a stakeholder workshop including service users and carers. Adopting Participatory Design approaches, we co-designed the recruitment intervention with PPIR partners using a leaflet to advertise the PPIR in EQUIP and sent potential participants invitations with the leaflet (intervention group) or not (control group). Primary outcome was the proportion of patients enrolled in EQUIP. Secondary outcomes included the proportions of patients who positively responded to the trial invitation.
Results: Thirty-four community mental health teams were randomised and 8182 service users invited. For the primary outcome, 4% of patients in the PPIR group were enrolled versus 5.3% of the control group. The intervention was not effective for improving recruitment rates (adjusted OR = 0.75, 95% CI = 0.53 to 1.07, p = 0.113). For the secondary outcome of positive response, the intervention was not effective, with 7.3% of potential participants in the intervention group responding positively versus 7.9% of the control group (adjusted OR = 0.74, 95% CI = 0.53 to 1.04, p = 0.082). We did not find a positive impact of directly advertising PPIR on any other outcomes.
Conclusion: To our knowledge, this is the largest ever embedded trial to evaluate a recruitment or PPIR intervention. Advertising PPIR did not improve enrolment rates or any other outcome. It is possible that rather than advertising PPIR being the means to improve recruitment, PPIR may have an alternative impact on trials by making them more attractive, acceptable and patient-centred. We discuss potential reasons for our findings and implications for recruitment practice and research.
Trial registration numbers: ISRCTN, ISRCTN16488358 . Registered on 14 May 2014. Study Within A Trial, SWAT-26 . Registered on 21 January 2016.
Keywords: Mesh: embedded trial; Patient and public involvement; Randomised controlled trial; Recruitment; Research methodology; Service user involvement; Study within a trial.
Figures
References
-
- CenterWatch. State of the clinical trials industry 2009: a sourcebook of charts and statistics. Boston: CenterWatch; 2009.
-
- Olsen K, Howel D, Barber R, Ford GA, Gallagher P, McAllister-Williams RH, Nilsson J, O’Brien J, Parker J, Thomas A. Lessons from a pilot and feasibility randomised trial in depression (Blood pressure Rapid Intensive Lowering And Normal Treatment for Mood and cognition in persistent depression (BRILiANT mood study)) Pilot Feasibility Stud. 2015;1(1):1–11. doi: 10.1186/s40814-015-0042-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

