Gene-environment interactions in cortical interneuron development and dysfunction: A review of preclinical studies
- PMID: 27932026
- PMCID: PMC5328258
- DOI: 10.1016/j.neuro.2016.12.002
Gene-environment interactions in cortical interneuron development and dysfunction: A review of preclinical studies
Abstract
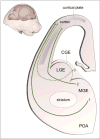
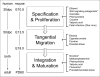
Cortical interneurons (cINs) are a diverse group of locally projecting neurons essential to the organization and regulation of neural networks. Though they comprise only ∼20% of neurons in the neocortex, their dynamic modulation of cortical activity is requisite for normal cognition and underlies multiple aspects of learning and memory. While displaying significant morphological, molecular, and electrophysiological variability, cINs collectively function to maintain the excitatory-inhibitory balance in the cortex by dampening hyperexcitability and synchronizing activity of projection neurons, primarily through use of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA). Disruption of the excitatory-inhibitory balance is a common pathophysiological feature of multiple seizure and neuropsychiatric disorders, including epilepsy, schizophrenia, and autism. While most studies have focused on genetic disruption of cIN development in these conditions, emerging evidence indicates that cIN development is exquisitely sensitive to teratogenic disruption. Here, we review key aspects of cIN development, including specification, migration, and integration into neural circuits. Additionally, we examine the mechanisms by which prenatal exposure to common chemical and environmental agents disrupt these events in preclinical models. Understanding how genetic and environmental factors interact to disrupt cIN development and function has tremendous potential to advance prevention and treatment of prevalent seizure and neuropsychiatric illnesses.
Keywords: Cortical development; Developmental neurotoxicity; Gene-environment; Interneuron; Schizophrenia; Seizure.
Copyright © 2016 Elsevier B.V. All rights reserved.
Conflict of interest statement
Disclosure Statement
The authors declare no conflict of interest.
Figures
References
-
- Dreifuss J, Kelly J, Krnjević K. Cortical inhibition and γ-aminobutyric acid. Experimental brain research. 1969;9:137–154. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

