The current state of therapeutic and T cell-based vaccines against human papillomaviruses
- PMID: 27932207
- PMCID: PMC5325765
- DOI: 10.1016/j.virusres.2016.12.002
The current state of therapeutic and T cell-based vaccines against human papillomaviruses
Abstract
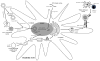
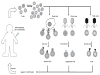
Human papillomavirus (HPV) is known to be a necessary factor for many gynecologic malignancies and is also associated with a subset of head and neck malignancies. This knowledge has created the opportunity to control these HPV-associated cancers through vaccination. However, despite the availability of prophylactic HPV vaccines, HPV infections remain extremely common worldwide. In addition, while prophylactic HPV vaccines have been effective in preventing infection, they are ineffective at clearing pre-existing HPV infections. Thus, there is an urgent need for therapeutic and T cell-based vaccines to treat existing HPV infections and HPV-associated lesions and cancers. Unlike prophylactic vaccines, which generate neutralizing antibodies, therapeutic, and T cell-based vaccines enhance cell-mediated immunity against HPV antigens. Our review will cover various therapeutic and T cell-based vaccines in development for the treatment of HPV-associated diseases. Furthermore, we review the strategies to enhance the efficacy of therapeutic vaccines and the latest clinical trials on therapeutic and T cell-based HPV vaccines.
Keywords: HPV E6; HPV E7; Human papillomavirus; Immunotherapy; T cell-based vaccine; Therapeutic vaccine.
Copyright © 2016 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declared that no potential conflict of interest exists.
Figures
Similar articles
-
Developments in L2-based human papillomavirus (HPV) vaccines.Virus Res. 2017 Mar 2;231:166-175. doi: 10.1016/j.virusres.2016.11.020. Epub 2016 Nov 23. Virus Res. 2017. PMID: 27889616 Free PMC article. Review.
-
Therapeutic DNA Vaccines for Human Papillomavirus and Associated Diseases.Hum Gene Ther. 2018 Sep;29(9):971-996. doi: 10.1089/hum.2017.197. Epub 2018 Mar 16. Hum Gene Ther. 2018. PMID: 29316817 Free PMC article.
-
Optimal induction of HPV DNA vaccine-induced CD8+ T cell responses and therapeutic antitumor effect by antigen engineering and electroporation.Vaccine. 2009 Sep 25;27(42):5906-12. doi: 10.1016/j.vaccine.2009.07.033. Epub 2009 Aug 3. Vaccine. 2009. PMID: 19651174
-
Human papillomavirus: current status and issues of vaccination.Arch Virol. 2014 Feb;159(2):199-205. doi: 10.1007/s00705-013-1827-z. Arch Virol. 2014. PMID: 24022639 Review.
-
Human papillomavirus vaccine against cervical cancer: Opportunity and challenge.Cancer Lett. 2020 Feb 28;471:88-102. doi: 10.1016/j.canlet.2019.11.039. Epub 2019 Dec 5. Cancer Lett. 2020. PMID: 31812696 Review.
Cited by
-
Design of a multi-epitope vaccine against cervical cancer using immunoinformatics approaches.Sci Rep. 2021 Jun 11;11(1):12397. doi: 10.1038/s41598-021-91997-4. Sci Rep. 2021. PMID: 34117331 Free PMC article.
-
Development of therapeutic vaccines for the treatment of diseases.Mol Biomed. 2022 Dec 8;3(1):40. doi: 10.1186/s43556-022-00098-9. Mol Biomed. 2022. PMID: 36477638 Free PMC article. Review.
-
The Interaction of Human Papillomavirus Infection and Prostaglandin E2 Signaling in Carcinogenesis: A Focus on Cervical Cancer Therapeutics.Cells. 2022 Aug 15;11(16):2528. doi: 10.3390/cells11162528. Cells. 2022. PMID: 36010605 Free PMC article. Review.
-
Arsenic exposure associated T cell proliferation, smoking, and vitamin D in Bangladeshi men and women.PLoS One. 2020 Jun 23;15(6):e0234965. doi: 10.1371/journal.pone.0234965. eCollection 2020. PLoS One. 2020. PMID: 32574193 Free PMC article.
-
Delayed vaccine-induced CD8+ T cell expansion by topoisomerase I inhibition mediates enhanced CD70-dependent tumor eradication.J Immunother Cancer. 2023 Nov 29;11(11):e007158. doi: 10.1136/jitc-2023-007158. J Immunother Cancer. 2023. PMID: 38030302 Free PMC article.
References
-
- Adachi K, Kawana K, Yokoyama T, Fujii T, Tomio A, Miura S, Tomio K, Kojima S, Oda K, Sewaki T, Yasugi T, Kozuma S, Taketani Y. Oral immunization with a Lactobacillus casei vaccine expressing human papillomavirus (HPV) type 16 E7 is an effective strategy to induce mucosal cytotoxic lymphocytes against HPV16 E7. Vaccine. 2010;28(16):2810–2817. - PubMed
-
- Adams M, Navabi H, Jasani B, Man S, Fiander A, Evans AS, Donninger C, Mason M. Dendritic cell (DC) based therapy for cervical cancer: use of DC pulsed with tumour lysate and matured with a novel synthetic clinically non-toxic double stranded RNA analogue poly [I]:poly [C(12)U] (Ampligen R) Vaccine. 2003;21(7–8):787–790. - PubMed
-
- Alvarez RD, Huh WK, Bae S, Lamb LS, Jr, Conner MG, Boyer J, Wang C, Hung CF, Sauter E, Paradis M, Adams EA, Hester S, Jackson BE, Wu TC, Trimble CL. A pilot study of pNGVL4a-CRT/E7(detox) for the treatment of patients with HPV16+ cervical intraepithelial neoplasia 2/3 (CIN2/3) Gynecol Oncol. 2016;140(2):245–252. - PMC - PubMed
-
- Baez-Astua A, Herraez-Hernandez E, Garbi N, Pasolli HA, Juarez V, Zur Hausen H, Cid-Arregui A. Low-dose adenovirus vaccine encoding chimeric hepatitis B virus surface antigen-human papillomavirus type 16 E7 proteins induces enhanced E7-specific antibody and cytotoxic T-cell responses. J Virol. 2005;79(20):12807–12817. - PMC - PubMed
-
- Bagarazzi ML, Yan J, Morrow MP, Shen X, Parker RL, Lee JC, Giffear M, Pankhong P, Khan AS, Broderick KE, Knott C, Lin F, Boyer JD, Draghia-Akli R, White CJ, Kim JJ, Weiner DB, Sardesai NY. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med. 2012;4(155):155ra138. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

