Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling
- PMID: 27932289
- PMCID: PMC5810417
- DOI: 10.1016/j.abb.2016.12.003
Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling
Abstract
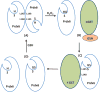
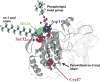
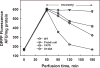
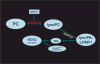
Peroxiredoxin 6 represents a widely distributed group of peroxiredoxins that contain a single conserved cysteine in the protein monomer (1-cys Prdx). The cys when oxidized to the sulfenic form is reduced with glutathione (GSH) catalyzed by the π isoform of GSH-S-transferase. Three enzymatic activities of the protein have been described:1) peroxidase with H2O2, short chain hydroperoxides, and phospholipid hydroperoxides as substrates; 2) phospholipase A2 (PLA2); and 3) lysophosphatidylcholine acyl transferase (LPCAT). These activities have important physiological roles in antioxidant defense, turnover of cellular phospholipids, and the generation of superoxide anion via initiation of the signaling cascade for activation of NADPH oxidase (type 2). The ability of Prdx6 to reduce peroxidized cell membrane phospholipids (peroxidase activity) and also to replace the oxidized sn-2 fatty acyl group through hydrolysis/reacylation (PLA2 and LPCAT activities) provides a complete system for the repair of peroxidized cell membranes.
Keywords: Anti-oxidant defense; Lysophospholipid acyl transferase; NADPH oxidase; Phospholipase A(2); Phospholipid hydroperoxide glutathione peroxidase; Phospholipid remodeling.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



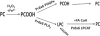
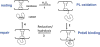


Similar articles
-
Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism.Free Radic Biol Med. 2005 Jun 1;38(11):1422-32. doi: 10.1016/j.freeradbiomed.2005.02.011. Free Radic Biol Med. 2005. PMID: 15890616 Review.
-
A novel lysophosphatidylcholine acyl transferase activity is expressed by peroxiredoxin 6.J Lipid Res. 2016 Apr;57(4):587-96. doi: 10.1194/jlr.M064758. Epub 2016 Feb 1. J Lipid Res. 2016. PMID: 26830860 Free PMC article.
-
Peroxiredoxin 6: a bifunctional enzyme with glutathione peroxidase and phospholipase A₂ activities.Antioxid Redox Signal. 2011 Aug 1;15(3):831-44. doi: 10.1089/ars.2010.3412. Epub 2011 Mar 31. Antioxid Redox Signal. 2011. PMID: 20919932 Free PMC article. Review.
-
Structural and Functional Diversity of the Peroxiredoxin 6 Enzyme Family.Antioxid Redox Signal. 2024 May;40(13-15):759-775. doi: 10.1089/ars.2023.0287. Epub 2023 Sep 12. Antioxid Redox Signal. 2024. PMID: 37463006 Review.
-
Peroxiredoxin 6 homodimerization and heterodimerization with glutathione S-transferase pi are required for its peroxidase but not phospholipase A2 activity.Free Radic Biol Med. 2016 May;94:145-56. doi: 10.1016/j.freeradbiomed.2016.02.012. Epub 2016 Feb 16. Free Radic Biol Med. 2016. PMID: 26891882 Free PMC article.
Cited by
-
Peroxiredoxin 6 alleviates high glucose-induced inflammation and apoptosis in HK-2 cells by inhibiting TLR4/NF-κB signaling.Ann Transl Med. 2023 Jan 31;11(2):41. doi: 10.21037/atm-22-6063. Epub 2023 Jan 9. Ann Transl Med. 2023. PMID: 36819569 Free PMC article.
-
Peroxiredoxin, Senescence, and Cancer.Cells. 2022 May 28;11(11):1772. doi: 10.3390/cells11111772. Cells. 2022. PMID: 35681467 Free PMC article. Review.
-
Proliferative Glioblastoma Cancer Cells Exhibit Persisting Temporal Control of Metabolism and Display Differential Temporal Drug Susceptibility in Chemotherapy.Mol Neurobiol. 2019 Feb;56(2):1276-1292. doi: 10.1007/s12035-018-1152-3. Epub 2018 Jun 7. Mol Neurobiol. 2019. PMID: 29881948
-
Peroxiredoxin 6 in Stress Orchestration and Disease Interplay.Antioxidants (Basel). 2025 Mar 23;14(4):379. doi: 10.3390/antiox14040379. Antioxidants (Basel). 2025. PMID: 40298631 Free PMC article. Review.
-
Modulation of Antioxidant Enzyme Expression of In Vitro Culture-Derived Reticulocytes.Antioxidants (Basel). 2024 Sep 2;13(9):1070. doi: 10.3390/antiox13091070. Antioxidants (Basel). 2024. PMID: 39334729 Free PMC article.
References
-
- Shichi H, Demar JC. Non-selenium glutathione peroxidase without gluta-thione S-transferase activity from bovine ciliary body. Experim Eye Res. 1990;50:513–520. - PubMed
-
- Peshenko IV, Novoselov VI, Evdokimov VA, Yu Nikolaev V, Shuvaeva TM, Lipkin VM, Fesenko EE. Novel 28-kDa secretory protein from rat olfactory epithelium. FEBS Lett. 1996;381:12–14. - PubMed
-
- Akiba S, Dodia C, Chen X, Fisher AB. Characterization of acidic Ca(2+)- independent phospholipase A2 of bovine lung. Comp Biochem Physiol B Biochem Mol Biol. 1998;120:393–404. - PubMed
-
- Peshenko IV, Novoselov VI, Evdokimov VA, Nikolaev YV, Kamzalov SS, Shuvaeva TM, Lipkin VM, Fesenko EE. Identification of a 28 kDa secretory protein from rat olfactory epithelium as a thiol-specific antioxidant. Free Radic Biol Med. 1998;25:654–659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

