Jasmonic Acid Enhances Al-Induced Root Growth Inhibition
- PMID: 27932419
- PMCID: PMC5291015
- DOI: 10.1104/pp.16.01756
Jasmonic Acid Enhances Al-Induced Root Growth Inhibition
Abstract
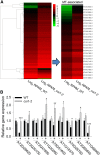
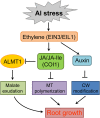
Phytohormones such as ethylene and auxin are involved in the regulation of the aluminum (Al)-induced root growth inhibition. Although jasmonate (JA) has been reported to play a crucial role in the regulation of root growth and development in response to environmental stresses through interplay with ethylene and auxin, its role in the regulation of root growth response to Al stress is not yet known. In an attempt to elucidate the role of JA, we found that exogenous application of JA enhanced the Al-induced root growth inhibition. Furthermore, phenotype analysis with mutants defective in either JA biosynthesis or signaling suggests that JA is involved in the regulation of Al-induced root growth inhibition. The expression of the JA receptor CORONATINE INSENSITIVE1 (COI1) and the key JA signaling regulator MYC2 was up-regulated in response to Al stress in the root tips. This process together with COI1-mediated Al-induced root growth inhibition under Al stress was controlled by ethylene but not auxin. Transcriptomic analysis revealed that many responsive genes under Al stress were regulated by JA signaling. The differential responsive of microtubule organization-related genes between the wild-type and coi1-2 mutant is consistent with the changed depolymerization of cortical microtubules in coi1 under Al stress. In addition, ALMT-mediated malate exudation and thus Al exclusion from roots in response to Al stress was also regulated by COI1-mediated JA signaling. Together, this study suggests that root growth inhibition is regulated by COI1-mediated JA signaling independent from auxin signaling and provides novel insights into the phytohormone-mediated root growth inhibition in response to Al stress.
© 2017 American Society of Plant Biologists. All Rights Reserved.
Figures







References
-
- Audic S, Claverie JM (1997) The significance of digital gene expression profiles. Genome Res 7: 986–995 - PubMed
-
- Baluška F, Brailsford RW, Hauskrecht M, Jackson MB, Barlow PW (1993) Cellular dimorphism in the maize root cortex: involvement of microtubules, ethylene and gibberellin in the differentiation of cellular behaviour in postmitotic growth zones. Plant Biol 106: 394–403
-
- Baluška F, Mancuso S, Volkmann D, Barlow PW (2010) Root apex transition zone: a signalling-response nexus in the root. Trends Plant Sci 15: 402–408 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

