Scn2b Deletion in Mice Results in Ventricular and Atrial Arrhythmias
- PMID: 27932425
- PMCID: PMC5161227
- DOI: 10.1161/CIRCEP.116.003923
Scn2b Deletion in Mice Results in Ventricular and Atrial Arrhythmias
Abstract
Background: Mutations in SCN2B, encoding voltage-gated sodium channel β2-subunits, are associated with human cardiac arrhythmias, including atrial fibrillation and Brugada syndrome. Because of this, we propose that β2-subunits play critical roles in the establishment or maintenance of normal cardiac electric activity in vivo.
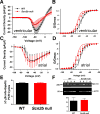
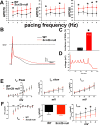
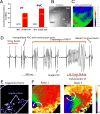
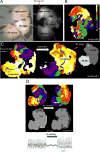
Methods and results: To understand the pathophysiological roles of β2 in the heart, we investigated the cardiac phenotype of Scn2b null mice. We observed reduced sodium and potassium current densities in ventricular myocytes, as well as conduction slowing in the right ventricular outflow tract region. Functional reentry, resulting from the interplay between slowed conduction, prolonged repolarization, and increased incidence of premature ventricular complexes, was found to underlie the mechanism of spontaneous polymorphic ventricular tachycardia. Scn5a transcript levels were similar in Scn2b null and wild-type ventricles, as were levels of Nav1.5 protein, suggesting that similar to the previous work in neurons, the major function of β2-subunits in the ventricle is to chaperone voltage-gated sodium channel α-subunits to the plasma membrane. Interestingly, Scn2b deletion resulted in region-specific effects in the heart. Scn2b null atria had normal levels of sodium current density compared with wild type. Scn2b null hearts were more susceptible to atrial fibrillation, had increased levels of fibrosis, and higher repolarization dispersion than wild-type littermates.
Conclusions: Genetic deletion of Scn2b in mice results in ventricular and atrial arrhythmias, consistent with reported SCN2B mutations in human patients.
Keywords: action potentials; atrial fibrillation; fibrosis; potassium channels; sodium channels.
© 2016 American Heart Association, Inc.
Figures



References
-
- Bao Y, Isom LL. Nav1.5 and regulatory β subunits in cardiac sodium channelopathies. Card Electrophysiol Clin. 2014;6:679–694.
-
- Riuro H, Beltran-Alvarez P, Tarradas A, Selga E, Campuzano O, Verges M, Pagans S, Iglesias A, Brugada J, Brugada P, Vazquez FM, Perez GJ, Scornik FS, Brugada R. A missense mutation in the sodium channel beta2 subunit reveals scn2b as a new candidate gene for brugada syndrome. Hum Mutat. 2013;34:961–966. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

