Roles and regulations of the ETS transcription factor ELF4/MEF
- PMID: 27932483
- PMCID: PMC5907832
- DOI: 10.1093/jmcb/mjw051
Roles and regulations of the ETS transcription factor ELF4/MEF
Abstract
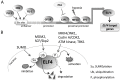
Most E26 transformation-specific (ETS) transcription factors are involved in the pathogenesis and progression of cancer. This is in part due to the roles of ETS transcription factors in basic biological processes such as growth, proliferation, and differentiation, and also because of their regulatory functions that have physiological relevance in tumorigenesis, immunity, and basal cellular homoeostasis. A member of the E74-like factor (ELF) subfamily of the ETS transcription factor family-myeloid elf-1-like factor (MEF), designated as ELF4-has been shown to be critically involved in immune response and signalling, osteogenesis, adipogenesis, cancer, and stem cell quiescence. ELF4 carries out these functions as a transcriptional activator or through interactions with its partner proteins. Mutations in ELF4 cause aberrant interactions and induce downstream processes that may lead to diseased cells. Knowing how ELF4 impinges on certain cellular processes and how it is regulated in the cells can lead to a better understanding of the physiological and pathological consequences of modulated ELF4 activity.
Keywords: ELF4; ETS transcription factors; cancer; immune regulation; myeloid elf-1-like factor (MEF); transcriptional regulation.
© The Author (2016). Published by Oxford University Press on behalf of Journal of Molecular Cell Biology, IBCB, SIBS, CAS.
Figures


References
-
- Ando M., Kawazu M., Ueno T., et al. (2016). Mutational landscape and antiproliferative functions of ELF transcription factors in human cancer. Cancer Res. 76, 1814–1824. - PubMed
-
- Aryee D.N., Petermann R., Kos K., et al. (1998). Cloning of a novel human ELF-1-related ETS transcription factor, ELFR, its characterization and chromosomal assignment relative to ELF-1. Gene 210, 71–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

