Computational modeling of epithelial fluid and ion transport in the parotid duct after transfection of human aquaporin-1
- PMID: 27932503
- PMCID: PMC5341129
- DOI: 10.1152/ajpgi.00374.2016
Computational modeling of epithelial fluid and ion transport in the parotid duct after transfection of human aquaporin-1
Abstract
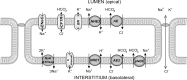
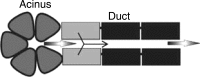
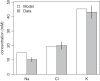
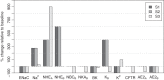
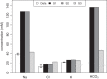
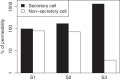
Previous studies have shown that localized delivery of the aquaporin-1 (AQP1) gene to the parotid duct can restore saliva flow in minipigs following irradiation-induced salivary hypofunction. The resulting flow rate and electrochemistry of secreted saliva contradicts current understanding of ductal fluid transport. We hypothesized that changes in expression of ion transport proteins have occurred following AQP1 transfection. We use a mathematical model of ion and fluid transport across the parotid duct epithelial cells to predict the expression profile of ion transporters that are consistent with the experimental measurements of saliva composition and secretion rates. Using a baseline set of parameters, the model reproduces the data for the irradiated, non-AQP1-transfected case. We propose three scenarios which may have occurred after transfection, which differ in the location of the AQP1 gene. The first scenario places AQP1 within nonsecretory cells, and requires that epithelial sodium channel (ENaC) expression is greatly reduced (1.3% of baseline), and ductal bicarbonate concentration is increased from 40.6 to 137.0 mM, to drive water secretion into the duct. The second scenario introduces the AQP1 gene into all ductal cells. The final scenario has AQP1 primarily in the proximal duct cells which secrete water under baseline conditions. We find the change in the remaining cells includes a 95.8% reduction in ENaC expression, enabling us to reproduce all experimental ionic concentrations within 9 mM. These findings provide a mechanistic basis for the observations and will guide the further development of gene transfer therapy for salivary hypofunction.
New & noteworthy: Following transfection of aquaporin into the parotid ducts of minipigs with salivary hypofunction, the resulting increase in salivary flow rates contradicts current understanding of ductal fluid transport. We show that the change in saliva electrochemistry and flow rate can be explained by changes in expression of ion transporters in the ductal cell membranes, using a mathematical model replicating a single parotid duct.
Keywords: aquaporin transfection; membrane transport; parotid duct.
Copyright © 2017 the American Physiological Society.
Figures



Similar articles
-
Transfer of the AQP1 cDNA for the correction of radiation-induced salivary hypofunction.Biochim Biophys Acta. 2006 Aug;1758(8):1071-7. doi: 10.1016/j.bbamem.2005.11.006. Epub 2005 Dec 5. Biochim Biophys Acta. 2006. PMID: 16368071 Review.
-
AAV2-mediated transfer of the human aquaporin-1 cDNA restores fluid secretion from irradiated miniature pig parotid glands.Gene Ther. 2011 Jan;18(1):38-42. doi: 10.1038/gt.2010.128. Epub 2010 Sep 30. Gene Ther. 2011. PMID: 20882054 Free PMC article.
-
Ultrasound-assisted nonviral gene transfer of AQP1 to the irradiated minipig parotid gland restores fluid secretion.Gene Ther. 2015 Sep;22(9):739-49. doi: 10.1038/gt.2015.36. Epub 2015 Apr 14. Gene Ther. 2015. PMID: 25871828 Free PMC article.
-
pH and bicarbonate excretion in the rat parotid gland as a function of salivary rate.Pflugers Arch. 1975 Apr 2;355(4):353-60. doi: 10.1007/BF00579856. Pflugers Arch. 1975. PMID: 1707
-
The first discovered water channel protein, later called aquaporin 1: molecular characteristics, functions and medical implications.Mol Aspects Med. 2012 Oct-Dec;33(5-6):518-34. doi: 10.1016/j.mam.2012.06.001. Epub 2012 Jun 15. Mol Aspects Med. 2012. PMID: 22705445 Review.
Cited by
-
Epithelial Na + Channels Function as Extracellular Sensors.Compr Physiol. 2024 Mar 29;14(2):1-41. doi: 10.1002/cphy.c230015. Compr Physiol. 2024. PMID: 39109974 Free PMC article. Review.
-
A Mathematical Model of Salivary Gland Duct Cells.Bull Math Biol. 2022 Jul 7;84(8):84. doi: 10.1007/s11538-022-01041-3. Bull Math Biol. 2022. PMID: 35799078 Free PMC article.
-
Computational Modeling of Glucose Uptake in the Enterocyte.Front Physiol. 2019 Apr 12;10:380. doi: 10.3389/fphys.2019.00380. eCollection 2019. Front Physiol. 2019. PMID: 31031632 Free PMC article.
-
Methods for studying mammalian aquaporin biology.Biol Methods Protoc. 2023 Nov 11;8(1):bpad031. doi: 10.1093/biomethods/bpad031. eCollection 2023. Biol Methods Protoc. 2023. PMID: 38046463 Free PMC article. Review.
-
Calcium Dynamics and Water Transport in Salivary Acinar Cells.Bull Math Biol. 2021 Feb 17;83(4):31. doi: 10.1007/s11538-020-00841-9. Bull Math Biol. 2021. PMID: 33594615 Free PMC article. Review.
References
-
- Benjamin BA, Johnson EA. A quantitative description of the Na-K-2Cl cotransporter and its conformity to experimental data. Am J Physiol Renal Physiol 273: F473–F482, 1997. - PubMed
-
- Blaug S, Hybiske K, Cohn J, Firestone GL, Machen TE, Miller SS. ENaC- and CFTR-dependent ion and fluid transport in mammary epithelia. Am J Physiol Cell Physiol 281: C633–C648, 2001. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

