Microfluidic analysis of oocyte and embryo biomechanical properties to improve outcomes in assisted reproductive technologies
- PMID: 27932552
- PMCID: PMC5909856
- DOI: 10.1093/molehr/gaw071
Microfluidic analysis of oocyte and embryo biomechanical properties to improve outcomes in assisted reproductive technologies
Abstract
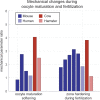
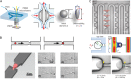
Measurement of oocyte and embryo biomechanical properties has recently emerged as an exciting new approach to obtain a quantitative, objective estimate of developmental potential. However, many traditional methods for probing cell mechanical properties are time consuming, labor intensive and require expensive equipment. Microfluidic technology is currently making its way into many aspects of assisted reproductive technologies (ART), and is particularly well suited to measure embryo biomechanics due to the potential for robust, automated single-cell analysis at a low cost. This review will highlight microfluidic approaches to measure oocyte and embryo mechanics along with their ability to predict developmental potential and find practical application in the clinic. Although these new devices must be extensively validated before they can be integrated into the existing clinical workflow, they could eventually be used to constantly monitor oocyte and embryo developmental progress and enable more optimal decision making in ART.
Keywords: biomechanics; embryo development; embryology; fertilization; microfluidics; oocyte maturation.
© The Author 2016. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Abadie J, Roux C, Piat E, Filiatre C, Amiot C. Experimental measurement of human oocyte mechanical properties on a micro and nanoforce sensing platform based on magnetic springs. Sens Actuators B Chem 2014;190:429–438.
-
- Bae CY, Kim MS, Park JK. Mechanical stimulation of bovine embryos in a microfluidic culture platform. Biochip J 2011;5:106–113.
-
- Balakier H, Tarkowski AK. Diploid parthenogenetic mouse embryos produced by heat-shock and cytochalasin B. J Embryol Exp Morphol 1976;35:25–39. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

