Amyloid Precursor Proteins Are Dynamically Trafficked and Processed during Neuronal Development
- PMID: 27932950
- PMCID: PMC5122739
- DOI: 10.3389/fnmol.2016.00130
Amyloid Precursor Proteins Are Dynamically Trafficked and Processed during Neuronal Development
Abstract
Proteolytic processing of the Amyloid Precursor Protein (APP) produces beta-amyloid (Aβ) peptide fragments that accumulate in Alzheimer's Disease (AD), but APP may also regulate multiple aspects of neuronal development, albeit via mechanisms that are not well understood. APP is a member of a family of transmembrane glycoproteins expressed by all higher organisms, including two mammalian orthologs (APLP1 and APLP2) that have complicated investigations into the specific activities of APP. By comparison, insects express only a single APP-related protein (APP-Like, or APPL) that contains the same protein interaction domains identified in APP. However, unlike its mammalian orthologs, APPL is only expressed by neurons, greatly simplifying an analysis of its functions in vivo. Like APP, APPL is processed by secretases to generate a similar array of extracellular and intracellular cleavage fragments, as well as an Aβ-like fragment that can induce neurotoxic responses in the brain. Exploiting the complementary advantages of two insect models (Drosophila melanogaster and Manduca sexta), we have investigated the regulation of APPL trafficking and processing with respect to different aspects of neuronal development. By comparing the behavior of endogenously expressed APPL with fluorescently tagged versions of APPL and APP, we have shown that some full-length protein is consistently trafficked into the most motile regions of developing neurons both in vitro and in vivo. Concurrently, much of the holoprotein is rapidly processed into N- and C-terminal fragments that undergo bi-directional transport within distinct vesicle populations. Unexpectedly, we also discovered that APPL can be transiently sequestered into an amphisome-like compartment in developing neurons, while manipulations targeting APPL cleavage altered their motile behavior in cultured embryos. These data suggest that multiple mechanisms restrict the bioavailability of the holoprotein to regulate APPL-dependent responses within the nervous system. Lastly, targeted expression of our double-tagged constructs (combined with time-lapse imaging) revealed that APP family proteins are subject to complex patterns of trafficking and processing that vary dramatically between different neuronal subtypes. In combination, our results provide a new perspective on how the regulation of APP family proteins can be modulated to accommodate a variety of cell type-specific responses within the embryonic and adult nervous system.
Keywords: APPL; D. melanogaster; M. sexta; amphisome; migration; outgrowth; secretase; transport.
Figures




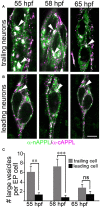
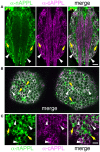
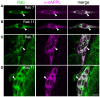
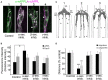




References
-
- Ashburner M. (1989). Drosophila. A Laboratory Handbook. New York, NY: Cold Spring Harbor Laboratory.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

