Proton Pump Inhibitors Display Antitumor Effects in Barrett's Adenocarcinoma Cells
- PMID: 27932981
- PMCID: PMC5122752
- DOI: 10.3389/fphar.2016.00452
Proton Pump Inhibitors Display Antitumor Effects in Barrett's Adenocarcinoma Cells
Abstract
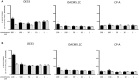
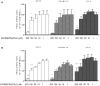
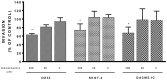
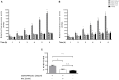
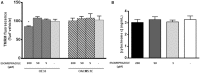
Recent evidence has reported that proton pump inhibitors (PPIs) can exert antineoplastic effects through the disruption of pH homeostasis by inhibiting vacuolar ATPase (H+-VATPase), a proton pump overexpressed in several tumor cells, but this aspect has not been deeply investigated in EAC yet. In the present study, the expression of H+-VATPase was assessed through the metaplasia-dysplasia-adenocarcinoma sequence in Barrett's esophagus (BE) and the antineoplastic effects of PPIs and cellular mechanisms involved were evaluated in vitro. H+-VATPase expression was assessed by immunohistochemistry in paraffined-embedded samples or by immunofluorescence in cultured BE and EAC cell lines. Cells were treated with different concentrations of PPIs and parameters of citotoxicity, oxidative stress, and autophagy were evaluated. H+-VATPase expression was found in all biopsies and cell lines evaluated, showing differences in the location of the pump between the cell lines. Esomeprazole inhibited proliferation and cell invasion and induced apoptosis of EAC cells. Production of reactive oxygen species (ROS) seemed to be involved in the cytotoxic effects observed since the addition of N-acetylcysteine significantly reduced esomeprazole-induced apoptosis in EAC cells. Esomeprazole also reduced intracellular pH of tumor cells, whereas only disturbed the mitochondrial membrane potential in OE33 cells. Esomeprazole induced autophagy in both EAC cells, but also triggered a blockade in autophagic flux in the metastatic cell line. These data provide in vitro evidence supporting the potential use of PPIs as novel antineoplastic drugs for EAC and also shed some light on the mechanisms that trigger PPIs cytotoxic effects, which differ upon the cell line evaluated.
Keywords: Barrett's esophagus; esophageal adenocarcinoma; proton pump inhibitors; reactive oxygen species; vacuolar ATPase.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources

