Efficacy and tolerability of repository corticotropin injection in patients with persistently active SLE: results of a phase 4, randomised, controlled pilot study
- PMID: 27933199
- PMCID: PMC5133412
- DOI: 10.1136/lupus-2016-000180
Efficacy and tolerability of repository corticotropin injection in patients with persistently active SLE: results of a phase 4, randomised, controlled pilot study
Abstract
Objective: To evaluate the efficacy of a prolonged-release formulation of a porcine adrenocorticotropic hormone analogue (repository corticotropin injection (RCI)) added to standard of care in patients requiring moderate-dose corticosteroids for symptomatic SLE.
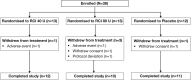
Methods: This prospective, randomised, double-blind, phase 4, pilot study (NCT01753401) enrolled 38 patients with persistently active SLE involving skin and/or joints. Enrolled patients received RCI, 40 U daily or 80 U every other day, or volume-matched placebo gel, for 8 weeks, with dose tapering to twice weekly during weeks 5-8. Efficacy endpoints included proportion of responders at week 4 based on a novel composite measure that included resolution of rash or arthritis measured using the hybrid SLE Disease Activity Index (hSLEDAI) without worsening British Isles Lupus Assessment Group (BILAG) scores in other organ systems at week 4 (primary), as well as improvements in total hSLEDAI and BILAG scores and other measures of skin and joint disease activity over the 8-week treatment period.
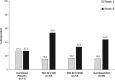
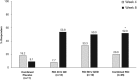
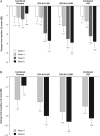
Results: Response, as defined for the primary endpoint, did not differ significantly between the combined placebo and RCI-treated groups at week 4. At week 8, the proportion of responders was higher in RCI-treated patients but did not statistically differ between groups (RCI 40 U (53.8%), RCI 80 U (33.3%), combined placebo (27.3%)). However, RCI treatment was associated with statistically significant improvements in several secondary endpoints, including total hSLEDAI, total BILAG and Cutaneous Lupus Erythematosus Disease Area and Severity Index Activity scores within 8 weeks. Treatment was well tolerated.
Conclusions: Although the primary endpoint was not met in this pilot study, secondary and post hoc analyses suggested that RCI was associated with improvements in SLE disease activity in a select patient population with steroid-dependent persistent disease.
Trial registration number: NCT01753401; results.
Keywords: Autoimmune Diseases; Corticosteroids; Disease Activity; Systemic Lupus Erythematosus.
Conflict of interest statement
RF has been a paid consultant to Mallinckrodt Pharmaceuticals as well as the study and a site principal investigator. MM is a paid consultant to Mallinckrodt Pharmaceuticals. EZ is an employee of Mallinckrodt Pharmaceuticals. MD and DL were employees of Mallinckrodt Pharmaceuticals at the time of the study and hold stock or stock options in the company. PMB is an employee of Mallinckrodt Pharmaceuticals and holds stock or stock options in the company.
Figures


References
-
- Tsokos GC. Systemic lupus erythematosus. N Engl J Med 2011;365:2110–21. doi:10.1056/NEJMra1100359 - DOI - PubMed
-
- Kasitanon N, Magder LS, Petri M. Predictors of survival in systemic lupus erythematosus. Medicine (Baltimore) 2006;85:147–56. doi:10.1097/01.md.0000224709.70133.f7 - DOI - PubMed
-
- Trager J, Ward MM. Mortality and causes of death in systemic lupus erythematosus. Curr Opin Rheumatol 2001;13:345–51. doi:10.1097/00002281-200109000-00002 - DOI - PubMed
-
- Holloway L, Humphrey L, Heron L et al. . Patient-reported outcome measures for systemic lupus erythematosus clinical trials: a review of content validity, face validity and psychometric performance. Health Qual Life Outcomes 2014;12:116 doi:10.1186/s12955-014-0116-1 - DOI - PMC - PubMed
-
- Petri M. Clinical research in systemic lupus erythematosus: immediate relevance to clinical practice. Int J Rheum Dis 2011;14:1–5. doi:10.1111/j.1756-185X.2010.01590.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
