Spontaneous destructive periodontitis and skeletal bone damage in transgenic mice carrying a human shared epitope-coding HLA-DRB1 allele
- PMID: 27933212
- PMCID: PMC5133411
- DOI: 10.1136/rmdopen-2016-000349
Spontaneous destructive periodontitis and skeletal bone damage in transgenic mice carrying a human shared epitope-coding HLA-DRB1 allele
Abstract
Objective: Shared epitope (SE)-coding DRB1 alleles are associated with bone erosion in several diseases, including rheumatoid arthritis (RA) and periodontal disease (PD), but the underlying mechanism is unknown. We have recently identified the SE as an osteoclast-activating ligand. To better understand the biological effects of the SE in vivo, here we sought to determine whether it can facilitate spontaneous bone damage in naïve mice.
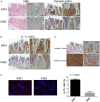
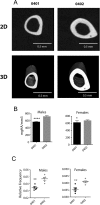
Methods: 3-month old naïve transgenic mice that carry the human SE-coding allele DRB1*04:01, or a SE-negative allele DRB1*04:02 were studied. Bone tissues were analysed by micro-CT, and the tooth-supporting tissues were studied by histology, immunohistochemistry and immunofluorescence. Serum biomarkers were determined by ELISA.
Results: Transgenic mice expressing the SE-coding DRB1*04:01 allele, but not mice carrying the SE-negative allele DRB1*04:02, showed spontaneous PD associated with interleukin (IL)-17 overabundance and periostin disruption. Mandibular bone volumetric and mineralisation parameters were significantly lower in SE-positive mice, and alveolar bone resorption was significantly increased in these mice. SE-positive mice also had more slender tibiae, and their marrow, cortical and total areas were lower than those of SE-negative mice. Additionally, significantly increased serum IL-17, tumour necrosis factor-α and osteoprotegrin levels were found in SE-positive mice, while their receptor activator of nuclear factor κ-B ligand levels were significantly lower.
Conclusions: A human SE-coding allele increases the propensity to spontaneous bone-destructive periodontal inflammation and skeletal bone damage in transgenic mice. These findings provide new insights into the previously documented but poorly understood association of the SE with accelerated bone erosion in RA and several other human diseases.
Keywords: Bone Mineral Density; Gene Polymorphism; Rheumatoid Arthritis.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

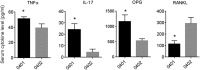
References
-
- da Silva AP, Bissada NF. “Arthritis and Periodontitis: an association debated for over two centuries”. Curr Rheumatol Rev Published Online First: 26 Oct 2015. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
