Genome-wide, high-content siRNA screening identifies the Alzheimer's genetic risk factor FERMT2 as a major modulator of APP metabolism
- PMID: 27933404
- PMCID: PMC5427165
- DOI: 10.1007/s00401-016-1652-z
Genome-wide, high-content siRNA screening identifies the Alzheimer's genetic risk factor FERMT2 as a major modulator of APP metabolism
Abstract
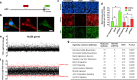
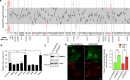
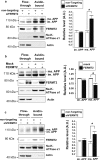
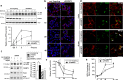
Genome-wide association studies (GWASs) have identified 19 susceptibility loci for Alzheimer's disease (AD). However, understanding how these genes are involved in the pathophysiology of AD is one of the main challenges of the "post-GWAS" era. At least 123 genes are located within the 19 susceptibility loci; hence, a conventional approach (studying the genes one by one) would not be time- and cost-effective. We therefore developed a genome-wide, high-content siRNA screening approach and used it to assess the functional impact of gene under-expression on APP metabolism. We found that 832 genes modulated APP metabolism. Eight of these genes were located within AD susceptibility loci. Only FERMT2 (a β3-integrin co-activator) was also significantly associated with a variation in cerebrospinal fluid Aβ peptide levels in 2886 AD cases. Lastly, we showed that the under-expression of FERMT2 increases Aβ peptide production by raising levels of mature APP at the cell surface and facilitating its recycling. Taken as a whole, our data suggest that FERMT2 modulates the AD risk by regulating APP metabolism and Aβ peptide production.
Conflict of interest statement
TM was a PhD student paid by SANOFI through a CIFRE contract.
Figures

Similar articles
-
Alzheimer's genetic risk factor FERMT2 (Kindlin-2) controls axonal growth and synaptic plasticity in an APP-dependent manner.Mol Psychiatry. 2021 Oct;26(10):5592-5607. doi: 10.1038/s41380-020-00926-w. Epub 2020 Nov 3. Mol Psychiatry. 2021. PMID: 33144711 Free PMC article.
-
Candidate-based screening via gene modulation in human neurons and astrocytes implicates FERMT2 in Aβ and TAU proteostasis.Hum Mol Genet. 2019 Mar 1;28(5):718-735. doi: 10.1093/hmg/ddy376. Hum Mol Genet. 2019. PMID: 30371777 Free PMC article.
-
Amyloid beta interacts with the amyloid precursor protein: a potential toxic mechanism in Alzheimer's disease.Nat Neurosci. 2000 May;3(5):460-4. doi: 10.1038/74833. Nat Neurosci. 2000. PMID: 10769385
-
Regulation of Alzheimer beta-amyloid precursor trafficking and metabolism.Biochim Biophys Acta. 2000 Jul 26;1502(1):44-52. doi: 10.1016/s0925-4439(00)00031-4. Biochim Biophys Acta. 2000. PMID: 10899430 Review.
-
Understanding the function of ABCA7 in Alzheimer's disease.Biochem Soc Trans. 2015 Oct;43(5):920-3. doi: 10.1042/BST20150105. Biochem Soc Trans. 2015. PMID: 26517904 Review.
Cited by
-
Genetic meta-analysis of diagnosed Alzheimer's disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing.Nat Genet. 2019 Mar;51(3):414-430. doi: 10.1038/s41588-019-0358-2. Epub 2019 Feb 28. Nat Genet. 2019. PMID: 30820047 Free PMC article.
-
Integrating amyloid and tau imaging with proteomics and genomics in Alzheimer's disease.Cell Rep Med. 2024 Sep 17;5(9):101735. doi: 10.1016/j.xcrm.2024.101735. Cell Rep Med. 2024. PMID: 39293391 Free PMC article. Review.
-
Regulation of the hippocampal translatome by Apoer2-ICD release.Mol Neurodegener. 2023 Sep 19;18(1):62. doi: 10.1186/s13024-023-00652-1. Mol Neurodegener. 2023. PMID: 37726747 Free PMC article.
-
Pyk2 overexpression in postsynaptic neurons blocks amyloid β1-42-induced synaptotoxicity in microfluidic co-cultures.Brain Commun. 2020 Aug 28;2(2):fcaa139. doi: 10.1093/braincomms/fcaa139. eCollection 2020. Brain Commun. 2020. PMID: 33718872 Free PMC article.
-
Sex-dependent autosomal effects on clinical progression of Alzheimer's disease.Brain. 2020 Jul 1;143(7):2272-2280. doi: 10.1093/brain/awaa164. Brain. 2020. PMID: 32591829 Free PMC article.
References
-
- Baranger K, Marchalant Y, Bonnet AE, Crouzin N, Carrete A, Paumier J-M, et al. MT5-MMP is a new pro-amyloidogenic proteinase that promotes amyloid pathology and cognitive decline in a transgenic mouse model of Alzheimer’s disease. Cell Mol Life Sci. 2016;73:217–236. doi: 10.1007/s00018-015-1992-1. - DOI - PMC - PubMed
-
- Bray M-A, Carpenter A, Imaging Platform BI of M and H (2013) Advanced assay development guidelines for image-based high content screening and analysis. Eli Lilly & Company and the National Center for Advancing Translational Sciences - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

