Gliogenic LTP spreads widely in nociceptive pathways
- PMID: 27934764
- PMCID: PMC6145441
- DOI: 10.1126/science.aah5715
Gliogenic LTP spreads widely in nociceptive pathways
Abstract
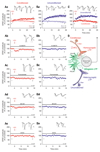
Learning and memory formation involve long-term potentiation (LTP) of synaptic strength. A fundamental feature of LTP induction in the brain is the need for coincident pre- and postsynaptic activity. This restricts LTP expression to activated synapses only (homosynaptic LTP) and leads to its input specificity. In the spinal cord, we discovered a fundamentally different form of LTP that is induced by glial cell activation and mediated by diffusible, extracellular messengers, including d-serine and tumor necrosis factor (TNF), and that travel long distances via the cerebrospinal fluid, thereby affecting susceptible synapses at remote sites. The properties of this gliogenic LTP resolve unexplained findings of memory traces in nociceptive pathways and may underlie forms of widespread pain hypersensitivity.
Copyright © 2016, American Association for the Advancement of Science.
Conflict of interest statement
The authors declare no conflicts of interest. M.T.K., R.D.-S., and J.S. designed the research. M.T.K., R.D.-S., M.G., S.D.H., and H.L.T. generated and analyzed the data. M.T.K., R.D.-S., and J.S. wrote the paper, with input from the other authors.
Figures
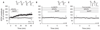


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

