Zika virus produces noncoding RNAs using a multi-pseudoknot structure that confounds a cellular exonuclease
- PMID: 27934765
- PMCID: PMC5476369
- DOI: 10.1126/science.aah3963
Zika virus produces noncoding RNAs using a multi-pseudoknot structure that confounds a cellular exonuclease
Abstract
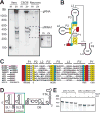
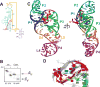
The outbreak of Zika virus (ZIKV) and associated fetal microcephaly mandates efforts to understand the molecular processes of infection. Related flaviviruses produce noncoding subgenomic flaviviral RNAs (sfRNAs) that are linked to pathogenicity in fetal mice. These viruses make sfRNAs by co-opting a cellular exonuclease via structured RNAs called xrRNAs. We found that ZIKV-infected monkey and human epithelial cells, mouse neurons, and mosquito cells produce sfRNAs. The RNA structure that is responsible for ZIKV sfRNA production forms a complex fold that is likely found in many pathogenic flaviviruses. Mutations that disrupt the structure affect exonuclease resistance in vitro and sfRNA formation during infection. The complete ZIKV xrRNA structure clarifies the mechanism of exonuclease resistance and identifies features that may modulate function in diverse flaviviruses.
Copyright © 2016, American Association for the Advancement of Science.
Figures


References
-
- Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nature medicine. 2004;10:S98–109. - PubMed
-
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika Virus. The New England journal of medicine. 2016;374:1552–1563. - PubMed
-
- Lindenbach BD, Thiel HJ, Rice CM. In: Fields Virology. 5. Knipe DM, Howley PM, editors. 2007.
-
- Pijlman GP, et al. A highly structured, nuclease-resistant, noncoding RNA produced by flaviviruses is required for pathogenicity. Cell host & microbe. 2008;4:579–591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

