Chiral Sulfoxide-Induced Single Turn Peptide α-Helicity
- PMID: 27934919
- PMCID: PMC5146914
- DOI: 10.1038/srep38573
Chiral Sulfoxide-Induced Single Turn Peptide α-Helicity
Abstract
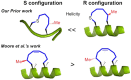
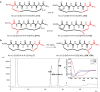
Inducing α-helicity through side-chain cross-linking is a strategy that has been pursued to improve peptide conformational rigidity and bio-availability. Here we describe the preparation of small peptides tethered to chiral sulfoxide-containing macrocyclic rings. Furthermore, a study of structure-activity relationships (SARs) disclosed properties with respect to ring size, sulfur position, oxidation state, and stereochemistry that show a propensity to induce α-helicity. Supporting data include circular dichroism spectroscopy (CD), NMR spectroscopy, and a single crystal X-ray structure for one such stabilized peptide. Finally, theoretical studies are presented to elucidate the effect of chiral sulfoxides in inducing backbone α-helicity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

