An isogenic blood-brain barrier model comprising brain endothelial cells, astrocytes, and neurons derived from human induced pluripotent stem cells
- PMID: 27935037
- PMCID: PMC5339046
- DOI: 10.1111/jnc.13923
An isogenic blood-brain barrier model comprising brain endothelial cells, astrocytes, and neurons derived from human induced pluripotent stem cells
Abstract
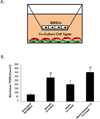
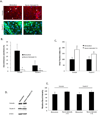
The blood-brain barrier (BBB) is critical in maintaining a physical and metabolic barrier between the blood and the brain. The BBB consists of brain microvascular endothelial cells (BMECs) that line the brain vasculature and combine with astrocytes, neurons and pericytes to form the neurovascular unit. We hypothesized that astrocytes and neurons generated from human-induced pluripotent stem cells (iPSCs) could induce BBB phenotypes in iPSC-derived BMECs, creating a robust multicellular human BBB model. To this end, iPSCs were used to form neural progenitor-like EZ-spheres, which were in turn differentiated to neurons and astrocytes, enabling facile neural cell generation. The iPSC-derived astrocytes and neurons induced barrier tightening in primary rat BMECs indicating their BBB inductive capacity. When co-cultured with human iPSC-derived BMECs, the iPSC-derived neurons and astrocytes significantly elevated trans-endothelial electrical resistance, reduced passive permeability, and improved tight junction continuity in the BMEC cell population, while p-glycoprotein efflux transporter activity was unchanged. A physiologically relevant neural cell mixture of one neuron: three astrocytes yielded optimal BMEC induction properties. Finally, an isogenic multicellular BBB model was successfully demonstrated employing BMECs, astrocytes, and neurons from the same donor iPSC source. It is anticipated that such an isogenic facsimile of the human BBB could have applications in furthering understanding the cellular interplay of the neurovascular unit in both healthy and diseased humans. Read the Editorial Highlight for this article on page 843.
Keywords: astrocytes; blood-brain barrier model; neurons; neurovascular unit; stem cells.
© 2016 International Society for Neurochemistry.
Conflict of interest statement
The authors declare there are no conflicts of interest.
Figures




Comment in
-
Blood-brain barrier models derived from individual patients: a new frontier: An Editorial Highlight on 'An isogenic blood-brain barrier model comprising brain endothelial cells, astrocytes, and neurons derived from human induced pluripotent stem cells'.J Neurochem. 2017 Mar;140(6):843-844. doi: 10.1111/jnc.13961. Epub 2017 Feb 14. J Neurochem. 2017. PMID: 28194778
References
-
- Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–541. - PubMed
-
- Berezowski V, Landry C, Dehouck MP, Cecchelli R, Fenart L. Contribution of glial cells and pericytes to the mRNA profiles of P-glycoprotein and multidrug resistance-associated proteins in an in vitro model of the blood-brain barrier. Brain Res. 2004;1018:1–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

