Quantification of ATP7B Protein in Dried Blood Spots by Peptide Immuno-SRM as a Potential Screen for Wilson's Disease
- PMID: 27935710
- PMCID: PMC5574172
- DOI: 10.1021/acs.jproteome.6b00828
Quantification of ATP7B Protein in Dried Blood Spots by Peptide Immuno-SRM as a Potential Screen for Wilson's Disease
Abstract
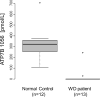
Wilson's Disease (WD), a copper transport disorder caused by a genetic defect in the ATP7B gene, has been a long time strong candidate for newborn screening (NBS), since early interventions can give better results by preventing irreversible neurological disability or liver cirrhosis. Several previous pilot studies measuring ceruloplasmin (CP) in infants or children showed that this marker alone was insufficient to meet the universal screening for WD. WD results from mutations that cause absent or markedly diminished levels of ATP7B. Therefore, ATP7B could serve as a marker for the screening of WD, if the protein can be detected from dried blood spots (DBS). This study demonstrates that the immuno-SRM platform can quantify ATP7B in DBS in the picomolar range, and that the assay readily distinguishes affected cases from normal controls (p < 0.0001). The assay precision was <10% CV, and the protein was stable for a week in DBS at room temperature. These promising proof-of-concept data open up the possibility of screening WD in newborns and the potential for a multiplexed assay for screening a variety of congenital disorders using proteins as biomarkers in DBS.
Keywords: ATP7B; DBS; NBS; WD; Wilson’s disease; dried blood spots; immuno-SRM; mass spectrometry; newborn screening; peptide immunoaffinity enrichment.
Conflict of interest statement
Conflict of interest statement: The authors have declared that no conflict of interest exists.
Figures





Similar articles
-
Population screening for Wilson's disease.Ann N Y Acad Sci. 2014 May;1315:64-9. doi: 10.1111/nyas.12423. Epub 2014 Apr 14. Ann N Y Acad Sci. 2014. PMID: 24731025 Review.
-
Rapid Multiplexed Proteomic Screening for Primary Immunodeficiency Disorders From Dried Blood Spots.Front Immunol. 2018 Dec 4;9:2756. doi: 10.3389/fimmu.2018.02756. eCollection 2018. Front Immunol. 2018. PMID: 30564228 Free PMC article.
-
Direct sequencing of mutations in the copper-transporting P-type adenosine triphosphate (ATP7B) gene for diagnosis and pathogenesis of Wilson's disease.Genet Mol Res. 2016 Sep 23;15(3). doi: 10.4238/gmr.15038746. Genet Mol Res. 2016. PMID: 27706781
-
p.H1069Q mutation in ATP7B and biochemical parameters of copper metabolism and clinical manifestation of Wilson's disease.Mov Disord. 2006 Feb;21(2):245-8. doi: 10.1002/mds.20671. Mov Disord. 2006. PMID: 16211609
-
Psychiatric Symptoms in Wilson's Disease-Consequence of ATP7B Gene Mutations or Just Coincidence?-Possible Causal Cascades and Molecular Pathways.Int J Mol Sci. 2024 Nov 18;25(22):12354. doi: 10.3390/ijms252212354. Int J Mol Sci. 2024. PMID: 39596417 Free PMC article. Review.
Cited by
-
Direct Measurement of ATP7B Peptides Is Highly Effective in the Diagnosis of Wilson Disease.Gastroenterology. 2021 Jun;160(7):2367-2382.e1. doi: 10.1053/j.gastro.2021.02.052. Epub 2021 Feb 25. Gastroenterology. 2021. PMID: 33640437 Free PMC article.
-
Recent advances in Wilson disease.Transl Gastroenterol Hepatol. 2021 Apr 5;6:21. doi: 10.21037/tgh-2020-02. eCollection 2021. Transl Gastroenterol Hepatol. 2021. PMID: 33824925 Free PMC article. Review.
-
Advancing Newborn Screening in Washington State: A Novel Multiplexed LC-MS/MS Proteomic Assay for Wilson Disease and Inborn Errors of Immunity.Int J Neonatal Screen. 2025 Jan 10;11(1):6. doi: 10.3390/ijns11010006. Int J Neonatal Screen. 2025. PMID: 39846592 Free PMC article.
-
Case reports of metabolic disorders from Nepal.Mol Genet Metab Rep. 2019 Nov 19;21:100542. doi: 10.1016/j.ymgmr.2019.100542. eCollection 2019 Dec. Mol Genet Metab Rep. 2019. PMID: 31788425 Free PMC article.
-
Targeted Mass Spectrometry Enables Multiplexed Quantification of Immunomodulatory Proteins in Clinical Biospecimens.Front Immunol. 2021 Nov 11;12:765898. doi: 10.3389/fimmu.2021.765898. eCollection 2021. Front Immunol. 2021. PMID: 34858420 Free PMC article.
References
-
- Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993;5:327. - PubMed
-
- Petrukhin K, Fischer SG, Pirastu M, Tanzi RE, Chernov I, Devoto M, Brzustowicz LM, Cayanis E, Vitale E, Russo JJ, et al. Mapping, cloning and genetic characterization of the region containing the Wilson disease gene. Nat Genet. 1993;5:338. - PubMed
-
- Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowicz LM, et al. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet. 1993;5:344. - PubMed
-
- Scheinberg IH, Gitlin D. Deficiency of ceruloplasmin in patients with hepatolenticular degeneration (Wilson’s disease) Science. 1952;116:484. - PubMed
-
- Lutsenko S, Efremov RG, Tsivkovskii R, Walker JM. Human copper-transporting ATPase ATP7B (the Wilson’s disease protein): biochemical properties and regulation. J Bioenerg Biomembr. 2002;34:351. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

