Designing and analysing clinical trials in mental health: an evidence synthesis approach
- PMID: 27935810
- PMCID: PMC10699521
- DOI: 10.1136/eb-2016-102491
Designing and analysing clinical trials in mental health: an evidence synthesis approach
Abstract
Objective: When planning a clinical study, evidence on the treatment effect is often available from previous studies. However, this evidence is mostly ignored for the analysis of the new study. This is unfortunate, since using it could lead to a smaller study without compromising power. We describe a design that addresses this issue.
Methods: We use a Bayesian meta-analytic model to incorporate the available evidence in the analysis of the new study. The shrinkage estimate for the new study integrates the evidence from the other studies. At the planning phase of the study, it allows a statistically justified reduction of the sample size.
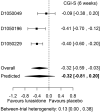
Results: The design is illustrated using data from an Food and Drug Administration (FDA) review of lurasidone for the treatment of schizophrenia. Three studies inform the meta-analysis before the new study is conducted. Results from an additional phase III study, which were not available at the time of the FDA review, are then used for the actual analysis.
Conclusions: In the presence of reliable and relevant evidence, the design offers a way to conduct a smaller study without compromising power. It therefore fills a gap between the assessment of evidence and its actual use in the design and analysis of studies.
Keywords: MENTAL HEALTH; STATISTICS & RESEARCH METHODS.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Figures

Similar articles
-
The INVEST project: investigating the use of evidence synthesis in the design and analysis of clinical trials.Trials. 2017 May 15;18(1):219. doi: 10.1186/s13063-017-1955-y. Trials. 2017. PMID: 28506284 Free PMC article.
-
Medication-Induced Akathisia with Newly Approved Antipsychotics in Patients with a Severe Mental Illness: A Systematic Review and Meta-Analysis.CNS Drugs. 2019 Jun;33(6):549-566. doi: 10.1007/s40263-019-00625-3. CNS Drugs. 2019. PMID: 31065941
-
Lurasidone in the treatment of schizophrenia: a critical evaluation.Expert Opin Pharmacother. 2015;16(10):1559-65. doi: 10.1517/14656566.2015.1058780. Expert Opin Pharmacother. 2015. PMID: 26111577 Review.
-
Identifying the genetic risk factors for treatment response to lurasidone by genome-wide association study: A meta-analysis of samples from three independent clinical trials.Schizophr Res. 2018 Sep;199:203-213. doi: 10.1016/j.schres.2018.04.006. Epub 2018 May 2. Schizophr Res. 2018. PMID: 29730043
-
Lurasidone for the acute treatment of adults with schizophrenia: what is the number needed to treat, number needed to harm, and likelihood to be helped or harmed?Clin Schizophr Relat Psychoses. 2012 Jul;6(2):76-85. doi: 10.3371/CSRP.6.2.5. Clin Schizophr Relat Psychoses. 2012. PMID: 22776634 Clinical Trial.
Cited by
-
Enhancing pediatric clinical trial feasibility through the use of Bayesian statistics.Pediatr Res. 2017 Nov;82(5):814-821. doi: 10.1038/pr.2017.163. Epub 2017 Aug 16. Pediatr Res. 2017. PMID: 28700566
-
Valproate for acute mania.Cochrane Database Syst Rev. 2019 Oct 7;10(10):CD004052. doi: 10.1002/14651858.CD004052.pub2. Cochrane Database Syst Rev. 2019. PMID: 31621892 Free PMC article. Review.
-
Relative to SSRI users, SSRI-statin users have fewer psychiatric hospital contacts and no increase in suicidal behaviour or all-cause mortality.Evid Based Ment Health. 2017 May;20(2):60. doi: 10.1136/eb-2016-102566. Epub 2017 Mar 10. Evid Based Ment Health. 2017. PMID: 28283544 Free PMC article. No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
