Feasibility of a novel one-stop ISET device to capture CTCs and its clinical application
- PMID: 27935872
- PMCID: PMC5356861
- DOI: 10.18632/oncotarget.13823
Feasibility of a novel one-stop ISET device to capture CTCs and its clinical application
Abstract
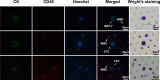
Introduction: Circulating tumor cells (CTCs) play a crucial role in cancer metastasis. In this study, we introduced a novel isolation method by size of epithelial tumor cells (ISET) device with automatic isolation and staining procedure, named one-stop ISET (osISET) and validated its feasibility to capture CTCs from cancer patients. Moreover, we aim to investigate the correlation between clinicopathologic features and CTCs in colorectal cancer (CRC) in order to explore its clinical application.
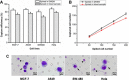
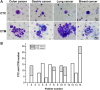
Results: The capture efficiency ranged from 80.3% to 88% with tumor cells spiked into medium while 67% to 78.3% with tumor cells spiked into healthy donors' blood. In detection blood samples of 72 CRC patients, CTCs and clusters of circulating tumor cells (CTC-clusters) were detected with a positive rate of 52.8% (38/72) and 18.1% (13/72) respectively. Moreover, CTC positive rate was associated with factors of lymphatic or venous invasion, tumor depth, lymph node metastasis and TNM stage in CRC patients (p < 0.01). Lymphocyte count and neutrophil to lymphocyte ratio (NLR) were significantly different between CTC positive and negative groups (p < 0.01).
Materials and methods: The capture efficiency of the device was tested by spiking cancer cells (MCF-7, A549, SW480, Hela) into medium or blood samples of healthy donors. Blood samples of 72 CRC patients were detected by osISET device. The clinicopathologic characteristics of 72 CRC patients were collected and the association with CTC positive rate or CTC count were analyzed.
Conclusions: Our osISET device was feasible to capture and identify CTCs and CTC-clusters from cancer patients. In addition, our device holds a potential for application in cancer management.
Keywords: circulating tumor cells (CTCs); clusters of circulating tumor cells (CTC-clusters); colorectal cancer (CRC); epithelial-mesenchymal transition (EMT); isolation method by size of epithelial tumor cells (ISET).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



Similar articles
-
Evaluation of the diagnostic value of circulating tumor cells with CytoSorter® CTC capture system in patients with breast cancer.Cancer Med. 2020 Mar;9(5):1638-1647. doi: 10.1002/cam4.2825. Epub 2020 Jan 6. Cancer Med. 2020. PMID: 31908156 Free PMC article.
-
Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches.J Thorac Oncol. 2012 Feb;7(2):306-15. doi: 10.1097/JTO.0b013e31823c5c16. J Thorac Oncol. 2012. PMID: 22173704
-
Classification of circulating tumor cells by epithelial-mesenchymal transition markers.PLoS One. 2015 Apr 24;10(4):e0123976. doi: 10.1371/journal.pone.0123976. eCollection 2015. PLoS One. 2015. PMID: 25909322 Free PMC article.
-
Recent advances and prospects in the isolation by size of epithelial tumor cells (ISET) methodology.Technol Cancer Res Treat. 2013 Aug;12(4):295-309. doi: 10.7785/tcrt.2012.500328. Epub 2013 Feb 22. Technol Cancer Res Treat. 2013. PMID: 23448577 Review.
-
Circulating Tumor Cells and Implications of the Epithelial-to-Mesenchymal Transition.Adv Clin Chem. 2018;83:121-181. doi: 10.1016/bs.acc.2017.10.004. Epub 2017 Dec 21. Adv Clin Chem. 2018. PMID: 29304900 Review.
Cited by
-
Circulating Tumour Cells: Detection and Application in Advanced Non-Small Cell Lung Cancer.Int J Mol Sci. 2023 Nov 8;24(22):16085. doi: 10.3390/ijms242216085. Int J Mol Sci. 2023. PMID: 38003273 Free PMC article. Review.
-
Circulating Hybrid Cells Join the Fray of Circulating Cellular Biomarkers.Cell Mol Gastroenterol Hepatol. 2019;8(4):595-607. doi: 10.1016/j.jcmgh.2019.07.002. Epub 2019 Jul 15. Cell Mol Gastroenterol Hepatol. 2019. PMID: 31319228 Free PMC article. Review.
-
Circulating Tumor Cells in Gastrointestinal Cancers: Current Status and Future Perspectives.Front Oncol. 2019 Dec 13;9:1427. doi: 10.3389/fonc.2019.01427. eCollection 2019. Front Oncol. 2019. PMID: 31921680 Free PMC article. Review.
-
Clinical Applications of Liquid Biopsy in Gastric Cancer.Front Med (Lausanne). 2021 Sep 28;8:749250. doi: 10.3389/fmed.2021.749250. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34651002 Free PMC article. Review.
-
Significant diagnostic value of circulating tumour cells in colorectal cancer.Oncol Lett. 2020 Jul;20(1):317-325. doi: 10.3892/ol.2020.11537. Epub 2020 Apr 15. Oncol Lett. 2020. PMID: 32565958 Free PMC article.
References
-
- Cristofanilli M. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. Semin Oncol. 2006;33:S9–14. - PubMed
-
- Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen LW. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218–4224. - PubMed
-
- Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse MA, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, et al. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol. 2009;20:1223–1229. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

