Intracellular Zn(II) Intoxication Leads to Dysregulation of the PerR Regulon Resulting in Heme Toxicity in Bacillus subtilis
- PMID: 27935957
- PMCID: PMC5189952
- DOI: 10.1371/journal.pgen.1006515
Intracellular Zn(II) Intoxication Leads to Dysregulation of the PerR Regulon Resulting in Heme Toxicity in Bacillus subtilis
Abstract
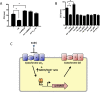
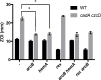
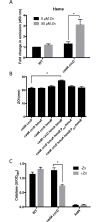
Transition metal ions (Zn(II), Cu(II)/(I), Fe(III)/(II), Mn(II)) are essential for life and participate in a wide range of biological functions. Cellular Zn(II) levels must be high enough to ensure that it can perform its essential roles. Yet, since Zn(II) binds to ligands with high avidity, excess Zn(II) can lead to protein mismetallation. The major targets of mismetallation, and the underlying causes of Zn(II) intoxication, are not well understood. Here, we use a forward genetic selection to identify targets of Zn(II) toxicity. In wild-type cells, in which Zn(II) efflux prevents intoxication of the cytoplasm, extracellular Zn(II) inhibits the electron transport chain due to the inactivation of the major aerobic cytochrome oxidase. This toxicity can be ameliorated by depression of an alternate oxidase or by mutations that restrict access of Zn(II) to the cell surface. Conversely, efflux deficient cells are sensitive to low levels of Zn(II) that do not inhibit the respiratory chain. Under these conditions, intracellular Zn(II) accumulates and leads to heme toxicity. Heme accumulation results from dysregulation of the regulon controlled by PerR, a metal-dependent repressor of peroxide stress genes. When metallated with Fe(II) or Mn(II), PerR represses both heme biosynthesis (hemAXCDBL operon) and the abundant heme protein catalase (katA). Metallation of PerR with Zn(II) disrupts this coordination, resulting in depression of heme biosynthesis but continued repression of catalase. Our results support a model in which excess heme partitions to the membrane and undergoes redox cycling catalyzed by reduced menaquinone thereby resulting in oxidative stress.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Irving H, Williams R. 637. The stability of transition-metal complexes. J Chem Soc. (Resumed). 1953: 3192–3210.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

