A Cost-Effective Method to Assemble Biomimetic 3D Cell Culture Platforms
- PMID: 27935982
- PMCID: PMC5147837
- DOI: 10.1371/journal.pone.0167116
A Cost-Effective Method to Assemble Biomimetic 3D Cell Culture Platforms
Abstract
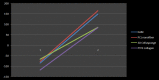
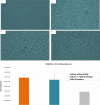
Methods: We utilized the hAM to provide the biological and the three dimensional (3D) topographic components of the prototype. The 3D nano-roughness of the hAM was characterized using surface electron microscopy and surface image analysis (ImageJ and SurfaceJ). We developed additional macro-scale and micro-scale versions of the platform which provided additional shear stress factors to simulate the fluid dynamics of the in vivo extracellular fluids.
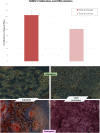
Results: Three models of varying complexities of the prototype were assembled. A well-defined 3D surface modulation of the hAM in comparable to commercial 3D biomaterial culture substrates was achieved without complex fabrication and with significantly lower cost. Performance of the prototype was demonstrated through culture of primary human umbilical cord mononuclear blood cells (MNCs), human bone marrow mesenchymal stem cell line (hBMSC), and human breast cancer tissue.
Conclusion: This study presents methods of assembling an integrated, flexible and low cost biomimetic cell culture platform for diverse cell culture applications.
Conflict of interest statement
We have declared in our first submission a filed patent related to this work. The patent was filed in June, 2015 as PCT/EG2015/000030 and has not been published yet. We confirm that this does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures



References
-
- Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Frontiers in immunology. 2012;3:297 Epub 2012/10/12. PubMed Central PMCID: PMC3458305. 10.3389/fimmu.2012.00297 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

