Long-Circulating Curcumin-Loaded Liposome Formulations with High Incorporation Efficiency, Stability and Anticancer Activity towards Pancreatic Adenocarcinoma Cell Lines In Vitro
- PMID: 27936114
- PMCID: PMC5147988
- DOI: 10.1371/journal.pone.0167787
Long-Circulating Curcumin-Loaded Liposome Formulations with High Incorporation Efficiency, Stability and Anticancer Activity towards Pancreatic Adenocarcinoma Cell Lines In Vitro
Erratum in
-
Correction: Long-Circulating Curcumin-Loaded Liposome Formulations with High Incorporation Efficiency, Stability and Anticancer Activity towards Pancreatic Adenocarcinoma Cell Lines In Vitro.PLoS One. 2017 Mar 8;12(3):e0173728. doi: 10.1371/journal.pone.0173728. eCollection 2017. PLoS One. 2017. PMID: 28273144 Free PMC article.
Abstract
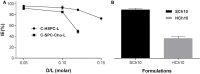
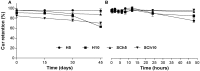
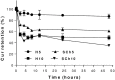
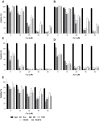
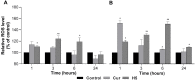
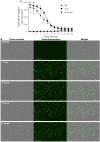
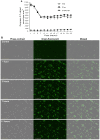
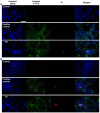
The incorporation of hydrophobic drugs into liposomes improve their bioavailability and leads to increased stability and anticancer activity, along with decreased drug toxicity. Curcumin (Cur) is a natural polyphenol compound with a potent anticancer activity in pancreatic adenocarcinoma (PA). In the present study, different types of Cur-loaded liposomal formulations were prepared and characterized in terms of size, shape, zeta potential, optimal drug-to-lipid ratio and stability at 4°C, 37°C; and in human plasma in vitro. The best formulation in terms of these parameters was PEGylated, cholesterol-free formulation based upon hydrogenated soya PC (HSPC:DSPE-PEG2000:Cur, termed H5), which had a 0.05/10 molar ratio of drug-to-lipid, was found to be stable and had a 96% Cur incorporation efficiency. All Cur-loaded liposomal formulations had potent anticancer activity on the PA cancer cell lines AsPC-1 and BxPC-3, and were less toxic to a normal cell line (NHDF). Furthermore, apoptosis-induction induced by Cur in PA cells was associated with morphological changes including cell shrinkage, cytoplasmic blebbing, irregularity in shape and the externalization of cell membrane phosphatidylserine, which was preceded by an increase in intracellular reactive oxygen species (ROS) generation and caspase 3/7 activation. Because the liposomal formulations tested here, especially the H5 variant which exhibited slow release of the Cur in the human plasma test, the formulation may be stable enough to facilitate the accumulation of pharmacologically active amounts of Cur in target cancer tissue by EPR. Therefore, our formulations could serve as a promising therapeutic approach for pancreatic cancer and other cancers.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Paclitaxel and curcumin coadministration in novel cationic PEGylated niosomal formulations exhibit enhanced synergistic antitumor efficacy.J Nanobiotechnology. 2018 Mar 23;16(1):28. doi: 10.1186/s12951-018-0351-4. J Nanobiotechnology. 2018. PMID: 29571289 Free PMC article.
-
Curcumin-guided nanotherapy: a lipid-based nanomedicine for targeted drug delivery in breast cancer therapy.Drug Deliv. 2016 May;23(4):1420-5. doi: 10.3109/10717544.2015.1066902. Epub 2015 Jul 23. Drug Deliv. 2016. PMID: 26203688
-
Curcumin-encapsulated MePEG/PCL diblock copolymeric micelles: a novel controlled delivery vehicle for cancer therapy.Nanomedicine (Lond). 2010 Apr;5(3):433-49. doi: 10.2217/nnm.10.9. Nanomedicine (Lond). 2010. PMID: 20394536
-
Curcumin AntiCancer Studies in Pancreatic Cancer.Nutrients. 2016 Jul 16;8(7):433. doi: 10.3390/nu8070433. Nutrients. 2016. PMID: 27438851 Free PMC article. Review.
-
Curcumin: New Insights into an Ancient Ingredient against Cancer.Int J Mol Sci. 2019 Apr 12;20(8):1808. doi: 10.3390/ijms20081808. Int J Mol Sci. 2019. PMID: 31013694 Free PMC article. Review.
Cited by
-
Encapsulation of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor in liposomes prepared by thin film hydration and their transfer to mesenchymal stem cells and cord blood hematopoietic stem cells.Arch Med Sci. 2020 Apr 18;18(4):1051-1061. doi: 10.5114/aoms.2020.94527. eCollection 2022. Arch Med Sci. 2020. PMID: 35832713 Free PMC article.
-
Innovative Approaches to Enhancing the Biomedical Properties of Liposomes.Pharmaceutics. 2024 Nov 27;16(12):1525. doi: 10.3390/pharmaceutics16121525. Pharmaceutics. 2024. PMID: 39771504 Free PMC article. Review.
-
The Comparison of In Vitro Photosensitizing Efficacy of Curcumin-Loaded Liposomes Following Photodynamic Therapy on Melanoma MUG-Mel2, Squamous Cell Carcinoma SCC-25, and Normal Keratinocyte HaCaT Cells.Pharmaceuticals (Basel). 2021 Apr 17;14(4):374. doi: 10.3390/ph14040374. Pharmaceuticals (Basel). 2021. PMID: 33920669 Free PMC article.
-
Preparation of curcuminoid microemulsions from Curcuma longa L. to enhance inhibition effects on growth of colon cancer cells HT-29.RSC Adv. 2018 Jan 9;8(5):2323-2337. doi: 10.1039/c7ra12297g. eCollection 2018 Jan 9. RSC Adv. 2018. PMID: 35541476 Free PMC article.
-
Curcumin Mitigates AFB1-Induced Hepatic Toxicity by Triggering Cattle Antioxidant and Anti-inflammatory Pathways: A Whole Transcriptomic In Vitro Study.Antioxidants (Basel). 2020 Oct 29;9(11):1059. doi: 10.3390/antiox9111059. Antioxidants (Basel). 2020. PMID: 33137966 Free PMC article.
References
-
- Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, et al. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nature Reviews Gastroenterology & Hepatology. Nature Publishing Group; 2012;9: 454–467. - PubMed
-
- Kanai M, Guha S, B. B. The Potential Role of Curcumin for Treatment of Pancreatic Cancer. Pancreatic Cancer—Molecular Mechanism and Targets. InTech; 2012.
-
- Tod J, Jenei V, Thomas G, Fine D. Tumor-stromal interactions in pancreatic cancer. Pancreatology. 2013;13: 1–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

