Immunolocalization of a Histidine-Rich Epidermal Differentiation Protein in the Chicken Supports the Hypothesis of an Evolutionary Developmental Link between the Embryonic Subperiderm and Feather Barbs and Barbules
- PMID: 27936131
- PMCID: PMC5147990
- DOI: 10.1371/journal.pone.0167789
Immunolocalization of a Histidine-Rich Epidermal Differentiation Protein in the Chicken Supports the Hypothesis of an Evolutionary Developmental Link between the Embryonic Subperiderm and Feather Barbs and Barbules
Abstract
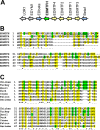
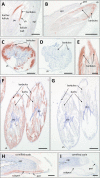
The morphogenesis of feathers is a complex process that depends on a tight spatiotemporal regulation of gene expression and assembly of the protein components of mature feathers. Recent comparative genomics and gene transcription studies have indicated that genes within the epidermal differentiation complex (EDC) encode numerous structural proteins of cornifying skin cells in amniotes including birds. Here, we determined the localization of one of these proteins, termed EDMTFH (Epidermal Differentiation Protein starting with a MTF motif and rich in Histidine), which belongs to a group of EDC-encoded proteins rich in aromatic amino acid residues. We raised an antibody against an EDMTFH-specific epitope and performed immunohistochemical investigations by light microscopy and immunogold labeling by electron microscopy of chicken embryos at days 14-18 of development. EDMTFH was specifically present in the subperiderm, a transient layer of the embryonic epidermis, and in barbs and barbules of feathers. In the latter, it partially localized to bundles of so-called feather beta-keratins (corneous beta-proteins, CBPs). Cells of the embryonic periderm, the epidermis proper, and the feather sheath were immunonegative for EDMTFH. The results of this study indicate that EDMTFH may contribute to the unique mechanical properties of feathers and define EDMTFH as a common marker of the subperiderm and the feather barbules. This expression pattern of EDMTFH resembles that of epidermal differentiation cysteine-rich protein (EDCRP) and feather CBPs and is in accordance with the hypothesis that a major part of the cyclically regenerating feather follicle is topologically, developmentally and evolutionarily related to the embryonic subperiderm.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

