Antibodies to Staphylococcus aureus capsular polysaccharides 5 and 8 perform similarly in vitro but are functionally distinct in vivo
- PMID: 27936346
- PMCID: PMC5626342
- DOI: 10.1080/21505594.2016.1270494
Antibodies to Staphylococcus aureus capsular polysaccharides 5 and 8 perform similarly in vitro but are functionally distinct in vivo
Abstract
The capsular polysaccharide (CP) produced by Staphylococcus aureus is a virulence factor that allows the organism to evade uptake and killing by host neutrophils. Polyclonal antibodies to the serotype 5 (CP5) and type 8 (CP8) capsular polysaccharides are opsonic and protect mice against experimental bacteremia provoked by encapsulated staphylococci. Thus, passive immunotherapy using CP antibodies has been considered for the prevention or treatment of invasive antibiotic-resistant S. aureus infections. In this report, we generated monoclonal antibodies (mAbs) against S. aureus CP5 or CP8. Backbone specific mAbs reacted with native and O-deacetylated CPs, whereas O-acetyl specific mAbs reacted only with native CPs. Reference strains of S. aureus and a selection of clinical isolates reacted by colony immunoblot with the CP5 and CP8 mAbs in a serotype-specific manner. The mAbs mediated in vitro CP type-specific opsonophagocytic killing of S. aureus strains, and mice passively immunized with CP5 mAbs were protected against S. aureus bacteremia. Neither CP8-specific mAbs or polyclonal antibodies protected mice against bacteremia provoked by serotype 8 S. aureus clinical isolates, although these same antibodies did protect against a serotype 5 S. aureus strain genetically engineered to produce CP8. We detected soluble CP8 in culture supernatants of serotype 8 clinical isolates and in the plasma of infected animals. Serotype 5 S. aureus released significantly less soluble CP5 in vitro and in vivo. The release of soluble CP8 by S. aureus may contribute to the inability of CP8 vaccines or antibodies to protect against serotype 8 staphylococcal infections.
Keywords: bacteremia; capsular polysaccharide; monoclonal antibodies; staphylococcus aureus.
Figures



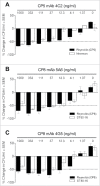

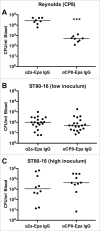
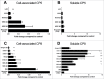

Comment in
-
Monoclonal antibodies and bacterial virulence.Virulence. 2017 Aug 18;8(6):635-636. doi: 10.1080/21505594.2017.1292199. Epub 2017 Feb 10. Virulence. 2017. PMID: 28276995 Free PMC article. No abstract available.
Similar articles
-
Antibodies to Staphylococcus aureus serotype 8 capsular polysaccharide react with and protect against serotype 5 and 8 isolates.Infect Immun. 2014 Dec;82(12):5049-55. doi: 10.1128/IAI.02373-14. Epub 2014 Sep 22. Infect Immun. 2014. PMID: 25245803 Free PMC article.
-
Staphylococcus aureus strains that express serotype 5 or serotype 8 capsular polysaccharides differ in virulence.Infect Immun. 2005 Jun;73(6):3502-11. doi: 10.1128/IAI.73.6.3502-3511.2005. Infect Immun. 2005. PMID: 15908379 Free PMC article.
-
Antibodies to capsular polysaccharide and clumping factor A prevent mastitis and the emergence of unencapsulated and small-colony variants of Staphylococcus aureus in mice.Infect Immun. 2008 Dec;76(12):5738-44. doi: 10.1128/IAI.00874-08. Epub 2008 Sep 22. Infect Immun. 2008. PMID: 18809660 Free PMC article.
-
Staphylococcus aureus capsular polysaccharides: a structural and synthetic perspective.Org Biomol Chem. 2020 Feb 7;18(5):783-798. doi: 10.1039/c9ob02546d. Epub 2020 Jan 10. Org Biomol Chem. 2020. PMID: 31922180 Review.
-
Staphylococcus aureus capsular polysaccharides.Clin Microbiol Rev. 2004 Jan;17(1):218-34. doi: 10.1128/CMR.17.1.218-234.2004. Clin Microbiol Rev. 2004. PMID: 14726462 Free PMC article. Review.
Cited by
-
Molecular epidemiology and expression of capsular polysaccharides in Staphylococcus aureus clinical isolates in the United States.PLoS One. 2019 Jan 14;14(1):e0208356. doi: 10.1371/journal.pone.0208356. eCollection 2019. PLoS One. 2019. PMID: 30641545 Free PMC article.
-
Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review.Pharmaceuticals (Basel). 2023 Nov 15;16(11):1615. doi: 10.3390/ph16111615. Pharmaceuticals (Basel). 2023. PMID: 38004480 Free PMC article. Review.
-
A novel bispecific antibody platform to direct complement activity for efficient lysis of target cells.Sci Rep. 2019 Aug 19;9(1):12031. doi: 10.1038/s41598-019-48461-1. Sci Rep. 2019. PMID: 31427700 Free PMC article.
-
Recognition of Staphylococcus aureus by the pattern recognition molecules langerin, mannan-binding lectin, and surfactant protein D: the influence of capsular polysaccharides and wall teichoic acid.Front Immunol. 2025 Jan 7;15:1504886. doi: 10.3389/fimmu.2024.1504886. eCollection 2024. Front Immunol. 2025. PMID: 39850879 Free PMC article.
-
Fighting Staphylococcus aureus Biofilms with Monoclonal Antibodies.Trends Microbiol. 2019 Apr;27(4):303-322. doi: 10.1016/j.tim.2018.12.009. Epub 2019 Jan 19. Trends Microbiol. 2019. PMID: 30665698 Free PMC article. Review.
References
-
- Scully IL, Liberator PA, Jansen KU, Anderson AS. Covering all the bases: Preclinical development of an effective Staphylococcus aureus vaccine. Front Immunol 2014; 5:109; PMID:24715889; http://dx.doi.org/10.3389/fimmu.2014.00109 - DOI - PMC - PubMed
-
- Joshi A, Pancari G, Cope L, Bowman EP, Cua D, Proctor RA, McNeely T. Immunization with Staphylococcus aureus iron regulated surface determinant B (IsdB) confers protection via Th17/IL17 pathway in a murine sepsis model. Hum Vaccin Immunother 2012; 8:336-46; PMID:22327491; http://dx.doi.org/10.4161/hv.18946 - DOI - PMC - PubMed
-
- Montgomery CP, Daniels M, Zhao F, Alegre ML, Chong AS, Daum RS. Protective immunity against recurrent Staphylococcus aureus skin infection requires antibody and interleukin-17A. Infect Immun 2014; 82:2125-34; PMID:24614654; http://dx.doi.org/10.1128/IAI.01491-14 - DOI - PMC - PubMed
-
- Yeaman MR, Filler SG, Chaili S, Barr K, Wang H, Kupferwasser D, Hennessey JP, Fu Y, Schmidt CS, Edwards JE, et al.. Mechanisms of NDV-3 vaccine efficacy in MRSA skin versus invasive infection. Proc Natl Acad Sci U S A 2014; 111:E5555-63; PMID:25489065; http://dx.doi.org/10.1073/pnas.1415610111 - DOI - PMC - PubMed
-
- Homaira N, Rawlinson W, Snelling TL, Jaffe A. Effectiveness of palivizumab in preventing RSV hospitalization in high risk children: a real-world perspective. Int J Pediatr 2014; 2014:571609; PMID:25548575; http://dx.doi.org/10.1155/2014/571609 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
