Refractory testicular germ cell tumors are highly sensitive to the second generation DNA methylation inhibitor guadecitabine
- PMID: 27936464
- PMCID: PMC5356854
- DOI: 10.18632/oncotarget.13811
Refractory testicular germ cell tumors are highly sensitive to the second generation DNA methylation inhibitor guadecitabine
Abstract
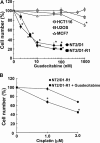
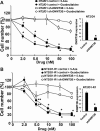
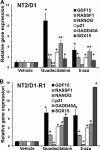
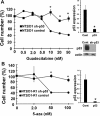
Testicular germ cell tumors (TGCTs) are the most common cancers of young males. A substantial portion of TGCT patients are refractory to cisplatin. There are no effective therapies for these patients, many of whom die from progressive disease. Embryonal carcinoma (EC) are the stem cells of TGCTs. In prior in vitro studies we found that EC cells were highly sensitive to the DNA methyltransferase inhibitor, 5-aza deoxycytidine (5-aza). Here, as an initial step in bringing demethylation therapy to the clinic for TGCT patients, we evaluated the effects of the clinically optimized, second generation demethylating agent guadecitabine (SGI-110) on EC cells in an animal model of cisplatin refractory testicular cancer. EC cells were exquisitely sensitive to guadecitabine and the hypersensitivity was dependent on high levels of DNA methyltransferase 3B. Guadecitabine mediated transcriptional reprogramming of EC cells included induction of p53 targets and repression of pluripotency genes. As a single agent, guadecitabine completely abolished progression and induced complete regression of cisplatin resistant EC xenografts even at doses well below those required to impact somatic solid tumors. Low dose guadecitabine also sensitized refractory EC cells to cisplatin in vivo. Genome-wide analysis indicated that in vivo antitumor activity was associated with activation of p53 and immune-related pathways and the antitumor effects of guadecitabine were dependent on p53, a gene rarely mutated in TGCTs. These preclinical findings suggest that guadecitabine alone or in combination with cisplatin is a promising strategy to treat refractory TGCT patients.
Keywords: DNA methylation; SGI-110; embryonal carcinoma; in vivo; testicular cancer.
Conflict of interest statement
P. Taverna is a Senior Director at Astex Pharmaceuticals. No potential conflicts of interest were disclosed by the other authors.
Figures



References
-
- Manecksha RP, Fitzpatrick JM. Epidemiology of testicular cancer. BJU Int. 2009;104:1329–1333. - PubMed
-
- Hanna NH, Einhorn LH. Testicular cancer—discoveries and updates. N Engl J Med. 2014;371:2005–2016. - PubMed
-
- Feldman DR, Patil S, Trinos MJ, Carousso M, Ginsberg MS, Sheinfeld J, Bajorin DF, Bosl GJ, Motzer RJ. Progression-free and overall survival in patients with relapsed/refractory germ cell tumors treated with single-agent chemotherapy: endpoints for clinical trial design. Cancer. 2012;118:981–986. - PubMed
-
- Porcu P, Bhatia S, Sharma M, Einhorn LH. Results of treatment after relapse from high-dose chemotherapy in germ cell tumors. J Clin Oncol. 2000;18:1181–1186. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

