T-bet over-expression regulates aryl hydrocarbon receptor-mediated T helper type 17 differentiation through an interferon (IFN)γ-independent pathway
- PMID: 27936495
- PMCID: PMC5343350
- DOI: 10.1111/cei.12912
T-bet over-expression regulates aryl hydrocarbon receptor-mediated T helper type 17 differentiation through an interferon (IFN)γ-independent pathway
Abstract
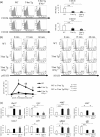
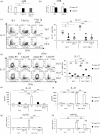
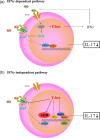
Various transcription factors are also known to enhance or suppress T helper type 17 (Th17) differentiation. We have shown previously that the development of collagen-induced arthritis was suppressed in T-bet transgenic (T-bet Tg) mice, and T-bet seemed to suppress Th17 differentiation through an interferon (IFN)-γ-independent pathway, although the precise mechanism remains to be clarified. The present study was designed to investigate further the mechanisms involved in the regulation of Th17 differentiation by T-bet over-expression, and we found the new relationship between T-bet and aryl hydrocarbon receptor (AHR). Both T-bet Tg mice and IFN-γ-/- -over-expressing T-bet (T-bet Tg/IFN-γ-/- ) mice showed inhibition of retinoic acid-related orphan receptor (ROR)γt expression and IL-17 production by CD4+ T cells cultured under conditions that promote Th-17 differentiation, and decreased IL-6 receptor (IL-6R) expression and signal transducer and activator of transcription-3 (STAT-3) phosphorylation in CD4+ T cells. The mRNA expression of ahr and rorc were suppressed in CD4+ T cells cultured under Th-17 conditions from T-bet Tg mice and T-bet Tg/IFN-γ-/- mice. CD4+ T cells of wild-type (WT) and IFN-γ-/- mice transduced with T-bet-expressing retrovirus also showed inhibition of IL-17 production, whereas T-bet transduction had no effect on IL-6R expression and STAT-3 phosphorylation. Interestingly, the mRNA expression of ahr and rorc were suppressed in CD4+ T cells with T-bet transduction cultured under Th17 conditions. The enhancement of interleukin (IL)-17 production from CD4+ T cells by the addition of AHR ligand with Th17 conditions was cancelled by T-bet over-expression. Our findings suggest that T-bet over-expression-induced suppression of Th17 differentiation is mediated through IFN-γ-independent AHR suppression.
Keywords: AHR; IL-17; T-bet over-expression.
© 2016 British Society for Immunology.
Figures




References
-
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T‐bet, directs Th1 lineage commitment. Cell 2000; 100:655–69. - PubMed
-
- Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T‐bet in TH1 lineage commitment and IFN‐γ production in CD4 and CD8 T cells. Science 2002; 295:338–42. - PubMed
-
- Afkarian M, Sedy JR, Yang J et al T‐bet is a STAT1‐induced regulator of IL‐12R expression in naïve CD4+ T cells. Nat Immunol 2002; 3:549–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

