Oxidative Cyclization in Natural Product Biosynthesis
- PMID: 27936626
- PMCID: PMC5406274
- DOI: 10.1021/acs.chemrev.6b00478
Oxidative Cyclization in Natural Product Biosynthesis
Abstract
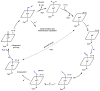
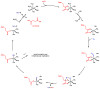
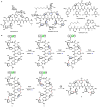
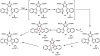
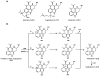
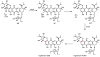
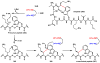
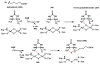
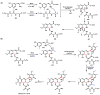
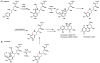
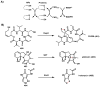
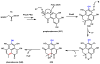
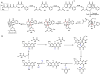
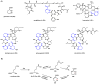
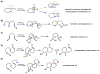
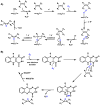
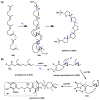
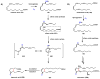
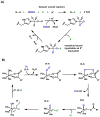
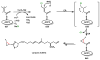
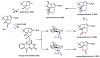
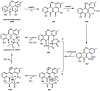
Oxidative cyclizations are important transformations that occur widely during natural product biosynthesis. The transformations from acyclic precursors to cyclized products can afford morphed scaffolds, structural rigidity, and biological activities. Some of the most dramatic structural alterations in natural product biosynthesis occur through oxidative cyclization. In this Review, we examine the different strategies used by nature to create new intra(inter)molecular bonds via redox chemistry. This Review will cover both oxidation- and reduction-enabled cyclization mechanisms, with an emphasis on the former. Radical cyclizations catalyzed by P450, nonheme iron, α-KG-dependent oxygenases, and radical SAM enzymes are discussed to illustrate the use of molecular oxygen and S-adenosylmethionine to forge new bonds at unactivated sites via one-electron manifolds. Nonradical cyclizations catalyzed by flavin-dependent monooxygenases and NAD(P)H-dependent reductases are covered to show the use of two-electron manifolds in initiating cyclization reactions. The oxidative installations of epoxides and halogens into acyclic scaffolds to drive subsequent cyclizations are separately discussed as examples of "disappearing" reactive handles. Last, oxidative rearrangement of rings systems, including contractions and expansions, will be covered.
Figures





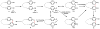

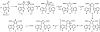
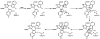
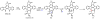


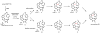
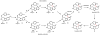

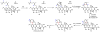

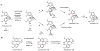
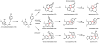
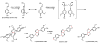

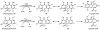
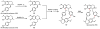


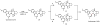



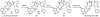
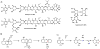
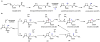
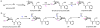



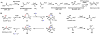


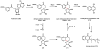





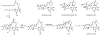
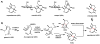

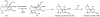

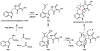
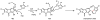
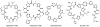
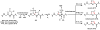


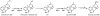


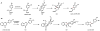
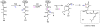
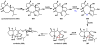

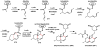

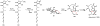

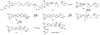




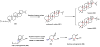
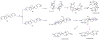


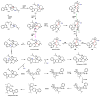




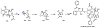
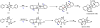



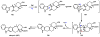


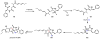

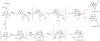



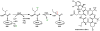
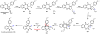




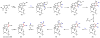
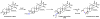





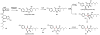
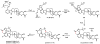





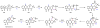


References
-
- Newman DJ, Cragg GM. Natural Products as Sources of New Drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629–661. - PubMed
-
- Mishra BB, Tiwari VK. Natural Products: An Evolving Role in Future Drug Discovery. Eur J Med Chem. 2011;46(10):4769–4807. - PubMed
-
- Walsh CT. A Chemocentric View of the Natural Product Inventory. Nat Chem Biol. 2015;11(9):620–624. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

