Validity of the Electrodiffusion Model for Calculating Conductance of Simple Ion Channels
- PMID: 27936743
- PMCID: PMC7517592
- DOI: 10.1021/acs.jpcb.6b09598
Validity of the Electrodiffusion Model for Calculating Conductance of Simple Ion Channels
Abstract
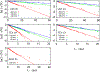
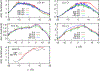
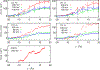
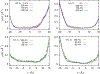
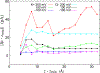
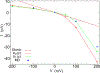
We examine the validity and utility of the electrodiffusion (ED) equation, i.e., the generalized Nernst-Planck equation, to characterize, in combination with molecular dynamics, the electrophysiological behavior of simple ion channels. As models, we consider three systems-two naturally occurring channels formed by α-helical bundles of peptaibols, trichotoxin, and alamethicin, and a synthetic, hexameric channel, formed by a peptide that contains only leucine and serine. All these channels mediate transport of potassium and chloride ions. Starting with equilibrium properties, such as the potential of mean force experienced by an ion traversing the channel and diffusivity, obtained from molecular dynamics simulations, the ED equation can be used to determine the full current-voltage dependence with modest or no additional effort. The potential of mean force can be obtained not only from equilibrium simulations, but also, with comparable accuracy, from nonequilibrium simulations at a single voltage. The main assumptions underlying the ED equation appear to hold well for the channels and voltages studied here. To expand the utility of the ED equation, we examine what are the necessary and sufficient conditions for Ohmic and nonrectifying behavior and relate deviations from this behavior to the shape of the ionic potential of mean force.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Combining molecular dynamics and an electrodiffusion model to calculate ion channel conductance.J Chem Phys. 2014 Dec 14;141(22):22D519. doi: 10.1063/1.4900879. J Chem Phys. 2014. PMID: 25494790
-
Computational Electrophysiology from a Single Molecular Dynamics Simulation and the Electrodiffusion Model.J Phys Chem B. 2021 Apr 1;125(12):3132-3144. doi: 10.1021/acs.jpcb.0c10737. Epub 2021 Mar 17. J Phys Chem B. 2021. PMID: 33729776
-
Effective diffusion coefficients of K+ and Cl- ions in ion channel models.Biophys Chem. 1999 Jun 7;79(2):129-51. doi: 10.1016/s0301-4622(99)00052-6. Biophys Chem. 1999. PMID: 10389238
-
Helical kink and channel behaviour: a comparative study with the peptaibols alamethicin, trichotoxin and antiamoebin.Eur Biophys J. 2004 May;33(3):169-74. doi: 10.1007/s00249-003-0383-y. Epub 2004 Mar 11. Eur Biophys J. 2004. PMID: 15014907 Review.
-
Model ion channels: gramicidin and alamethicin.J Membr Biol. 1992 Aug;129(2):109-36. doi: 10.1007/BF00219508. J Membr Biol. 1992. PMID: 1279177 Review.
Cited by
-
Fast bilayer-micelle fusion mediated by hydrophobic dipeptides.Biophys J. 2021 Jun 1;120(11):2330-2342. doi: 10.1016/j.bpj.2021.04.012. Epub 2021 Apr 19. Biophys J. 2021. PMID: 33887225 Free PMC article.
-
Opening of glutamate receptor channel to subconductance levels.Nature. 2022 May;605(7908):172-178. doi: 10.1038/s41586-022-04637-w. Epub 2022 Apr 20. Nature. 2022. PMID: 35444281 Free PMC article.
-
Electrophysiological Properties from Computations at a Single Voltage: Testing Theory with Stochastic Simulations.Entropy (Basel). 2021 May 6;23(5):571. doi: 10.3390/e23050571. Entropy (Basel). 2021. PMID: 34066581 Free PMC article.
-
Multi-oligomeric states of alamethicin ion channel: Assemblies and conductance.Biophys J. 2023 Jun 20;122(12):2531-2543. doi: 10.1016/j.bpj.2023.05.006. Epub 2023 May 8. Biophys J. 2023. PMID: 37161094 Free PMC article.
-
How to Connect Cardiac Excitation to the Atomic Interactions of Ion Channels.Biophys J. 2018 Jan 23;114(2):259-266. doi: 10.1016/j.bpj.2017.11.024. Biophys J. 2018. PMID: 29401425 Free PMC article. Review.
References
-
- Cheng MH; Coalson RD An accurate and efficient empirical approach for calculating the dielectric self-energy and ion-ion pair potential in continuum models of biological ion channels. J. Phys. Chem. B 2005, 109, 488–498. - PubMed
-
- Coalson RD; Kurnikova MG Poisson-Nernst-Planck theory approach to the calculation of current through biological ion channels. IEEE Trans. Nanobiosci 2005, 4, 81–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

