Altered expression of G1/S phase cell cycle regulators in placental mesenchymal stromal cells derived from preeclamptic pregnancies with fetal-placental compromise
- PMID: 27937072
- PMCID: PMC5283823
- DOI: 10.1080/15384101.2016.1261766
Altered expression of G1/S phase cell cycle regulators in placental mesenchymal stromal cells derived from preeclamptic pregnancies with fetal-placental compromise
Abstract
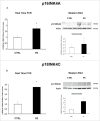
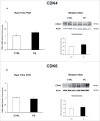
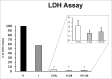
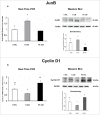
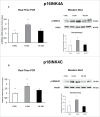
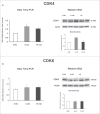
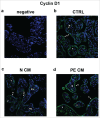
Herein, we evaluated whether Placental Mesenchymal Stromal Cells (PDMSCs) derived from normal and Preeclamptic (PE) placentae presented differences in the expression of G1/S-phase regulators p16INK4A, p18INK4C, CDK4 and CDK6. Finally, we investigated normal and PE-PDMSCs paracrine effects on JunB, Cyclin D1, p16INK4A, p18INK4C, CDK4 and CDK6 expressions in physiological term villous explants. PDMSCs were isolated from physiological (n = 20) and PE (n = 24) placentae. Passage three normal and PE-PDMSC and conditioned media (CM) were collected after 48h. Physiological villous explants (n = 60) were treated for 72h with normal or PE-PDMSCs CM. Explants viability was assessed by Lactate Dehydrogenase Cytotoxicity assay. Cyclin D1 localization was evaluated by Immuofluorescence (IF) while JunB, Cyclin-D1 p16INK4A, p18INK4C, CDK4 and CDK6 levels were assessed by Real Time PCR and Western Blot assay. We reported significantly increased p16INK4A and p18INK4C expression in PE- relative to normal PDMSCs while no differences in CDK4 and CDK6 levels were detected. Explants viability was not affected by normal or PE-PDMSCs CM. Normal PDMSCs CM increased JunB, p16INK4 and p18INK4C and decreased Cyclin-D1 in placental tissues. In contrast, PE-PDMSCs CM induced JunB downregulation and Cyclin D1 increase in placental explants. Cyclin D1 IF staining showed that CM treatment targeted mainly the syncytiotrophoblast. We showed Cyclin D1-p16INK4A/p18INK4C altered pathway in PE-PDMSCs demonstrating an aberrant G1/S phase transition in these pathological cells. The abnormal Cyclin D1-p16INK4A/p18INK4C expression in explants conditioned by PE-PDMSCs media suggest a key contribution of mesenchymal cells to the altered trophoblast cell cycle regulation typical of PE pregnancies with fetal-placental compromise.
Keywords: cell cycle; mesenchymal stromal cells; placenta; preeclampsia; pregnancy.
Figures
Similar articles
-
JunB/cyclin-D1 imbalance in placental mesenchymal stromal cells derived from preeclamptic pregnancies with fetal-placental compromise.Placenta. 2014 Jul;35(7):483-90. doi: 10.1016/j.placenta.2014.04.001. Epub 2014 Apr 14. Placenta. 2014. PMID: 24780198
-
Pro-inflammatory profile of preeclamptic placental mesenchymal stromal cells: new insights into the etiopathogenesis of preeclampsia.PLoS One. 2013;8(3):e59403. doi: 10.1371/journal.pone.0059403. Epub 2013 Mar 19. PLoS One. 2013. PMID: 23527185 Free PMC article.
-
Expression of p16Ink4a compensates for p18Ink4c loss in cyclin-dependent kinase 4/6-dependent tumors and tissues.Cancer Res. 2007 May 15;67(10):4732-41. doi: 10.1158/0008-5472.CAN-06-3437. Cancer Res. 2007. PMID: 17510401
-
[Molecular mechanisms controlling the cell cycle: fundamental aspects and implications for oncology].Cancer Radiother. 2001 Apr;5(2):109-29. doi: 10.1016/s1278-3218(01)00087-7. Cancer Radiother. 2001. PMID: 11355576 Review. French.
-
Alterations of pRb1-cyclin D1-cdk4/6-p16(INK4A) pathway in endometrial carcinogenesis.Cancer Lett. 2004 Jan 8;203(1):1-12. doi: 10.1016/j.canlet.2003.09.012. Cancer Lett. 2004. PMID: 14670612 Review.
Cited by
-
Mesenchymal Stem Cells as a Bio Organ for Treatment of Female Infertility.Cells. 2020 Oct 8;9(10):2253. doi: 10.3390/cells9102253. Cells. 2020. PMID: 33050021 Free PMC article. Review.
-
Effect of Placenta-Derived Mesenchymal Stromal Cells Conditioned Media on an LPS-Induced Mouse Model of Preeclampsia.Int J Mol Sci. 2022 Jan 31;23(3):1674. doi: 10.3390/ijms23031674. Int J Mol Sci. 2022. PMID: 35163594 Free PMC article.
-
Placental Ageing in Adverse Pregnancy Outcomes: Telomere Shortening, Cell Senescence, and Mitochondrial Dysfunction.Oxid Med Cell Longev. 2019 May 22;2019:3095383. doi: 10.1155/2019/3095383. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31249642 Free PMC article. Review.
-
Application of Mesenchymal Stem Cells in Female Infertility Treatment: Protocols and Preliminary Results.Life (Basel). 2024 Sep 13;14(9):1161. doi: 10.3390/life14091161. Life (Basel). 2024. PMID: 39337944 Free PMC article. Review.
-
Current Understanding of Autophagy in Pregnancy.Int J Mol Sci. 2019 May 11;20(9):2342. doi: 10.3390/ijms20092342. Int J Mol Sci. 2019. PMID: 31083536 Free PMC article. Review.
References
-
- Clarke B, Chetty R. Cell cycle aberrations in the pathogenesis of squamous cell carcinoma of the uterine cervix. Gynecologic Oncology 2001; 82:238-46; PMID:11531273; http://dx.doi.org/10.1006/gyno.2001.6306 - DOI - PubMed
-
- Milde-Langosch K, Riethdorf S. Role of cell-cycle regulatory proteins in gynecological cancer. Journal of Cellular Physiology 2003; 196:224-44; PMID:12811815; http://dx.doi.org/10.1002/jcp.10286 - DOI - PubMed
-
- Funk JO. Cancer cell cycle control. Anticancer Research 1999; 19:4772-80; PMID:10697591 - PubMed
-
- Rahat B, Hamid A, Ahmad Najar R, Bagga R, Kaur J. Epigenetic mechanisms regulate placental c-myc and hTERT in normal and pathological pregnancies; c-myc as a novel fetal DNA epigenetic marker for pre-eclampsia. Molecular human reproduction 2014; 20:1026-40; PMID:25024139; http://dx.doi.org/10.1093/molehr/gau053 - DOI - PubMed
-
- Strickland S, Richards WG. INVASION OF THE TROPHOBLASTS. Cell 1992; 71:355-7; PMID:1423599; http://dx.doi.org/10.1016/0092-8674(92)90503-5 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
