Novel Advances in Understanding of Molecular Pathogenesis of Hepatoblastoma: A Wnt/β-Catenin Perspective
- PMID: 27938502
- PMCID: PMC5311458
- DOI: 10.3727/105221616X693639
Novel Advances in Understanding of Molecular Pathogenesis of Hepatoblastoma: A Wnt/β-Catenin Perspective
Abstract
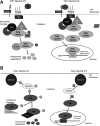
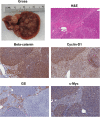
Hepatoblastoma is the most common pediatric liver malignancy, typically striking children within the first 3 years of their young lives. While advances in chemotherapy and newer surgical techniques have improved survival in patients with localized disease, unfortunately, for the 25% of patients with metastasis, the overall survival remains poor. These tumors, which are thought to arise from hepatic progenitors or hepatoblasts, hence the name hepatoblastoma, can be categorized by histological subtyping based on their level of cell differentiation. Genomic and histological analysis of human tumor samples has shown exon-3 deletions or missense mutations in gene coding for β-catenin, a downstream effector of the Wnt signaling pathway, in up to 90% of hepatoblastoma cases. The current article will review key aberrations in molecular pathways that are implicated in various subtypes of hepatoblastoma with an emphasis on Wnt signaling. It will also discuss cooperation among components of pathways such as β-catenin and Yes-associated protein in cancer development. Understanding the complex network of molecular signaling in oncogenesis will undoubtedly aid in the discovery of new therapeutics to help combat hepatoblastoma.
Figures



References
-
- Darbari A, Sabin KM, Shapiro CN, Schwarz KB. Epidemiology of primary hepatic malignancies in U.S. children. Hepatology 2003;38(3):560–6. - PubMed
-
- McLaughlin CC, Baptiste MS, Schymura MJ, Nasca PC, Zdeb MS. Maternal and infant birth characteristics and hepatoblastoma. Am J Epidemiol. 2006;163(9):818–28. - PubMed
-
- Czauderna P, Haeberle B, Hiyama E, Rangaswami A, Krailo M, Maibach R, Rinaldi E, Feng Y, Aronson D, Malogolowkin M, Yoshimura K, Leuschner I, Lopez-Terrada D, Hishiki T, Perilong G, von Schewinitz D, Schmid I, Watanabe K, Derosa M, Meyers R. The Children’s Hepatic tumors International Collaboration (CHIC): Novel global rare tumor database yields new prognostic factors in hepatoblastoma and becomes a research model. Eur J Cancer 2016;52:92–101. - PMC - PubMed
-
- Tanimura M, Matsui I, Abe J, Ikeda H, Kobayashi N, Ohira M, Yokoyama M, Kaneko M. Increased risk of hepatoblastoma among immature children with a lower birth weight. Cancer Res. 1998;58(14):3032–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
