Bile Duct Ligation Induces ATZ Globule Clearance in a Mouse Model of α-1 Antitrypsin Deficiency
- PMID: 27938510
- PMCID: PMC5296240
- DOI: 10.3727/105221616X692991
Bile Duct Ligation Induces ATZ Globule Clearance in a Mouse Model of α-1 Antitrypsin Deficiency
Abstract
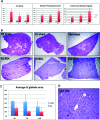
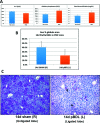
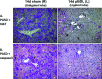
α-1 Antitrypsin deficiency (A1ATD) can progress to cirrhosis and hepatocellular carcinoma; however, not all patients are susceptible to severe liver disease. In A1ATD, a toxic gain-of-function mutation generates insoluble ATZ "globules" in hepatocytes, overwhelming protein clearance mechanisms. The relationship between bile acids and hepatocytic autophagy is less clear but may involve altered gene expression pathways. Based on previous findings that bile duct ligation (BDL) induces autophagy, we hypothesized that retained bile acids may have hepatoprotective effects in PiZZ transgenic mice, which model A1ATD. We performed BDL and partial BDL (pBDL) in PiZZ mice, followed by analysis of liver tissues. PiZZ liver subjected to BDL showed up to 50% clearance of ATZ globules, with increased expression of autophagy proteins. Analysis of transcription factors revealed significant changes. Surprisingly nuclear TFEB, a master regulator of autophagy, remained unchanged. pBDL confirmed that ATZ globule clearance was induced by localized stimuli rather than diet or systemic effects. Several genes involved in bile metabolism were overexpressed in globule-devoid hepatocytes, compared to globule-containing cells. Retained bile acids led to a dramatic reduction of ATZ globules, with enhanced hepatocyte regeneration and autophagy. These findings support investigation of synthetic bile acids as potential autophagy-enhancing agents.
Conflict of interest statement
Disclosures/Conflict of Interest: The authors do not have any disclosures or conflicts of interest.
Figures




Similar articles
-
Alpha-1 antitrypsin Z protein (PiZ) increases hepatic fibrosis in a murine model of cholestasis.Hepatology. 2007 Nov;46(5):1443-52. doi: 10.1002/hep.21832. Hepatology. 2007. PMID: 17668872
-
Analyses of hepatocellular proliferation in a mouse model of alpha-1-antitrypsin deficiency.Hepatology. 2004 Apr;39(4):1048-55. doi: 10.1002/hep.20118. Hepatology. 2004. PMID: 15057909
-
Gene transfer of master autophagy regulator TFEB results in clearance of toxic protein and correction of hepatic disease in alpha-1-anti-trypsin deficiency.EMBO Mol Med. 2013 Mar;5(3):397-412. doi: 10.1002/emmm.201202046. Epub 2013 Feb 4. EMBO Mol Med. 2013. PMID: 23381957 Free PMC article.
-
Pathogenesis of chronic liver injury and hepatocellular carcinoma in alpha-1-antitrypsin deficiency.Pediatr Res. 2006 Aug;60(2):233-8. doi: 10.1203/01.pdr.0000228350.61496.90. Pediatr Res. 2006. PMID: 16864711 Review.
-
Alpha-1 antitrypsin and liver disease: mechanisms of injury and novel interventions.Expert Rev Gastroenterol Hepatol. 2015 Feb;9(2):261-8. doi: 10.1586/17474124.2014.943187. Epub 2014 Jul 28. Expert Rev Gastroenterol Hepatol. 2015. PMID: 25066184 Review.
Cited by
-
Update on Alpha-1 Antitrypsin Deficiency in Liver Disease.Clin Liver Dis (Hoboken). 2020 Jun 30;15(6):228-235. doi: 10.1002/cld.896. eCollection 2020 Jun. Clin Liver Dis (Hoboken). 2020. PMID: 32617155 Free PMC article. Review. No abstract available.
-
Global 5'-UTR RNA structure regulates translation of a SERPINA1 mRNA.Nucleic Acids Res. 2022 Sep 23;50(17):9689-9704. doi: 10.1093/nar/gkac739. Nucleic Acids Res. 2022. PMID: 36107773 Free PMC article.
-
Liver Disease in Alpha-1 Antitrypsin Deficiency: Current Approaches and Future Directions.Curr Pathobiol Rep. 2017;5(3):243-252. doi: 10.1007/s40139-017-0147-5. Epub 2017 Jul 10. Curr Pathobiol Rep. 2017. PMID: 29399420 Free PMC article. Review.
-
Partial Bile Duct Ligation in the Mouse: A Controlled Model of Localized Obstructive Cholestasis.J Vis Exp. 2018 Mar 28;(133):56930. doi: 10.3791/56930. J Vis Exp. 2018. PMID: 29658929 Free PMC article.
-
Comparing animal well-being between bile duct ligation models.PLoS One. 2024 Jul 1;19(7):e0303786. doi: 10.1371/journal.pone.0303786. eCollection 2024. PLoS One. 2024. PMID: 38950046 Free PMC article.
References
-
- Teckman JH, An JK, Blomenkamp K, Schmidt B, Perlmutter D. Mitochondrial autophagy and injury in the liver in alpha 1-antitrypsin deficiency. Am J Physiol Gastrointest Liver Physiol. 2004;286(5):G851–62. - PubMed
-
- Piitulainen E, Carlson J, Ohlsson K, Sveger T. Alpha1-antitrypsin deficiency in 26-year-old subjects: Lung, liver, and protease/protease inhibitor studies. Chest 2005;128(4):2076–81. - PubMed
-
- Khan Z. Pathogenesis of alpha-1 antitrypsin deficiency in the liver: New approaches to old questions. J Liver Res Disord Ther. 2016;2(2):00023.
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
