Preclinical study of patient-specific cell-free nanofiber tissue-engineered vascular grafts using 3-dimensional printing in a sheep model
- PMID: 27938900
- PMCID: PMC5715716
- DOI: 10.1016/j.jtcvs.2016.10.066
Preclinical study of patient-specific cell-free nanofiber tissue-engineered vascular grafts using 3-dimensional printing in a sheep model
Abstract
Background: Tissue-engineered vascular grafts (TEVGs) offer potential to overcome limitations of current approaches for reconstruction in congenital heart disease by providing biodegradable scaffolds on which autologous cells proliferate and provide physiologic functionality. However, current TEVGs do not address the diverse anatomic requirements of individual patients. This study explores the feasibility of creating patient-specific TEVGs by combining 3-dimensional (3D) printing and electrospinning technology.
Methods: An electrospinning mandrel was 3D-printed after computer-aided design based on preoperative imaging of the ovine thoracic inferior vena cava (IVC). TEVG scaffolds were then electrospun around the 3D-printed mandrel. Six patient-specific TEVGs were implanted as cell-free IVC interposition conduits in a sheep model and explanted after 6 months for histologic, biochemical, and biomechanical evaluation.
Results: All sheep survived without complications, and all grafts were patent without aneurysm formation or ectopic calcification. Serial angiography revealed significant decreases in TEVG pressure gradients between 3 and 6 months as the grafts remodeled. At explant, the nanofiber scaffold was nearly completely resorbed and the TEVG showed similar mechanical properties to that of native IVC. Histological analysis demonstrated an organized smooth muscle cell layer, extracellular matrix deposition, and endothelialization. No significant difference in elastin and collagen content between the TEVG and native IVC was identified. There was a significant positive correlation between wall thickness and CD68+ macrophage infiltration into the TEVG.
Conclusions: Creation of patient-specific nanofiber TEVGs by combining electrospinning and 3D printing is a feasible technology as future clinical option. Further preclinical studies involving more complex anatomical shapes are warranted.
Keywords: 3D printing; Fontan circulation; cell-free tissue engineering; congenital heart disease; electrospun nanofibers; patient-specific; preclinical study; sheep model; tissue-engineered vascular graft.
Copyright © 2016. Published by Elsevier Inc.
Conflict of interest statement
Authors have nothing to disclose with regard to commercial support.
Figures
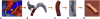

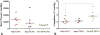



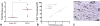
Comment in
-
It's in 3D, but is it truly the next dimension?J Thorac Cardiovasc Surg. 2017 Apr;153(4):923. doi: 10.1016/j.jtcvs.2016.10.046. Epub 2016 Nov 3. J Thorac Cardiovasc Surg. 2017. PMID: 27993363 No abstract available.
-
Recreating the inferior vena cava with a patient-specific biodegradable conduit.J Thorac Cardiovasc Surg. 2017 Apr;153(4):933. doi: 10.1016/j.jtcvs.2016.11.045. Epub 2016 Dec 5. J Thorac Cardiovasc Surg. 2017. PMID: 28109610 Free PMC article. No abstract available.
Similar articles
-
Tissue-Engineered Small Diameter Arterial Vascular Grafts from Cell-Free Nanofiber PCL/Chitosan Scaffolds in a Sheep Model.PLoS One. 2016 Jul 28;11(7):e0158555. doi: 10.1371/journal.pone.0158555. eCollection 2016. PLoS One. 2016. PMID: 27467821 Free PMC article.
-
Targeted imaging of matrix metalloproteinase activity in the evaluation of remodeling tissue-engineered vascular grafts implanted in a growing lamb model.J Thorac Cardiovasc Surg. 2014 Nov;148(5):2227-33. doi: 10.1016/j.jtcvs.2014.05.037. Epub 2014 May 21. J Thorac Cardiovasc Surg. 2014. PMID: 24952823 Free PMC article.
-
Role of Bone Marrow Mononuclear Cell Seeding for Nanofiber Vascular Grafts.Tissue Eng Part A. 2018 Jan;24(1-2):135-144. doi: 10.1089/ten.TEA.2017.0044. Epub 2017 Jun 13. Tissue Eng Part A. 2018. PMID: 28486019 Free PMC article.
-
Concise Review: Patency of Small-Diameter Tissue-Engineered Vascular Grafts: A Meta-Analysis of Preclinical Trials.Stem Cells Transl Med. 2019 Jul;8(7):671-680. doi: 10.1002/sctm.18-0287. Epub 2019 Mar 28. Stem Cells Transl Med. 2019. PMID: 30920771 Free PMC article.
-
Tissue engineered small-diameter vascular grafts.Clin Plast Surg. 2003 Oct;30(4):507-17. doi: 10.1016/s0094-1298(03)00069-5. Clin Plast Surg. 2003. PMID: 14621299 Review.
Cited by
-
The Evolution of Tissue Engineered Vascular Graft Technologies: From Preclinical Trials to Advancing Patient Care.Appl Sci (Basel). 2019 Apr;9(7):1274. doi: 10.3390/app9071274. Epub 2019 Mar 27. Appl Sci (Basel). 2019. PMID: 31890320 Free PMC article.
-
Patient-specific tissue engineered vascular graft for aortic arch reconstruction.JTCVS Open. 2024 Feb 27;18:209-220. doi: 10.1016/j.xjon.2024.02.012. eCollection 2024 Apr. JTCVS Open. 2024. PMID: 38690440 Free PMC article.
-
Validity of Customized Branched Tissue Engineered Vascular Graft in a Porcine Model.Ann Thorac Surg Short Rep. 2023 Jun 14;1(3):426-430. doi: 10.1016/j.atssr.2023.05.018. eCollection 2023 Sep. Ann Thorac Surg Short Rep. 2023. PMID: 39790945 Free PMC article.
-
Recent Advances in Hydrogel-Based 3D Bioprinting and Its Potential Application in the Treatment of Congenital Heart Disease.Biomolecules. 2024 Jul 18;14(7):861. doi: 10.3390/biom14070861. Biomolecules. 2024. PMID: 39062575 Free PMC article. Review.
-
Electrospun hybrid nanofibers: Fabrication, characterization, and biomedical applications.Front Bioeng Biotechnol. 2022 Dec 1;10:986975. doi: 10.3389/fbioe.2022.986975. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36561047 Free PMC article. Review.
References
-
- Dearani JA, Danielson GK, Puga FJ, Schaff HV, Warnes CW, Driscoll DJ, et al. Late follow-up of 1095 patients undergoing operation for complex congenital heart disease utilizing pulmonary ventricle to pulmonary artery conduits. Ann Thorac Surg. 2003;75:399–410. discussion 410–1. - PubMed
-
- Petrossian E, Reddy VM, McElhinney DB, Akkersdijk GP, Moore P, Parry AJ, et al. Early results of the extracardiac conduit Fontan operation. J Thorac Cardiovasc Surg. 1999;117:688–96. - PubMed
-
- Itatani K, Miyaji K, Tomoyasu T, Nakahata Y, Ohara K, Takamoto S, et al. Optimal conduit size of the extracardiac Fontan operation based on energy loss and flow stagnation. Ann Thorac Surg. 2009;88:565–72. discussion 572–3. - PubMed
-
- Naito Y, Imai Y, Shin’oka T, Kashiwagi J, Aoki M, Watanabe M, et al. Successful clinical application of tissue-engineered graft for extracardiac Fontan operation. J Thorac Cardiovasc Surg. 2003;125:419–20. - PubMed
-
- Shin’oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

