Adhesion-Dependent Wave Generation in Crawling Cells
- PMID: 27939309
- PMCID: PMC5225140
- DOI: 10.1016/j.cub.2016.11.011
Adhesion-Dependent Wave Generation in Crawling Cells
Abstract
Dynamic actin networks are excitable. In migrating cells, feedback loops can amplify stochastic fluctuations in actin dynamics, often resulting in traveling waves of protrusion. The precise contributions of various molecular and mechanical interactions to wave generation have been difficult to disentangle, in part due to complex cellular morphodynamics. Here we used a relatively simple cell type-the fish epithelial keratocyte-to define a set of mechanochemical feedback loops underlying actin network excitability and wave generation. Although keratocytes are normally characterized by the persistent protrusion of a broad leading edge, increasing cell-substrate adhesion strength results in waving protrusion of a short leading edge. We show that protrusion waves are due to fluctuations in actin polymerization rates and that overexpression of VASP, an actin anti-capping protein that promotes actin polymerization, switches highly adherent keratocytes from waving to persistent protrusion. Moreover, VASP localizes both to adhesion complexes and to the leading edge. Based on these results, we developed a mathematical model for protrusion waves in which local depletion of VASP from the leading edge by adhesions-along with lateral propagation of protrusion due to the branched architecture of the actin network and negative mechanical feedback from the cell membrane-results in regular protrusion waves. Consistent with our model simulations, we show that VASP localization at the leading edge oscillates, with VASP leading-edge enrichment greatest just prior to protrusion initiation. We propose that the mechanochemical feedbacks underlying wave generation in keratocytes may constitute a general module for establishing excitable actin dynamics in other cellular contexts.
Keywords: VASP; actin dynamics; actin waves; adhesion dynamics; cell motility; excitable system; keratocyte; leading edge.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures

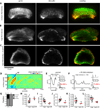
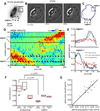

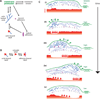
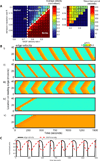
Comment in
-
Cell Migration: Making the Waves.Curr Biol. 2017 Jan 9;27(1):R24-R25. doi: 10.1016/j.cub.2016.11.035. Curr Biol. 2017. PMID: 28073017
References
-
- Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–1022. - PubMed
-
- Giannone G, Dubin-Thaler B, Dobereiner H-G, Kieffer N, Bresnick A, Sheetz M. Periodic lamellipodial contractions correlate with rearward actin waves. Cell. 2004;116:431–443. - PubMed
-
- Döbereiner H-G, Dubin-Thaler BJ, Hofman JM, Xenias HS, Sims TN, Giannone G, Dustin ML, Wiggins CH, Sheetz MP. Lateral membrane waves constitute a universal dynamic pattern of motile cells. Phys. Rev. Lett. 2006;97:038102. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

