Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial
- PMID: 27939400
- PMCID: PMC5568632
- DOI: 10.1016/S0140-6736(16)32455-2
Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial
Erratum in
-
Department of Error.Lancet. 2017 Aug 26;390(10097):848. doi: 10.1016/S0140-6736(17)32213-4. Epub 2017 Aug 11. Lancet. 2017. PMID: 28807535 No abstract available.
Abstract
Background: First-line chemotherapy for patients with cisplatin-ineligible locally advanced or metastatic urothelial carcinoma is associated with short response duration, poor survival, and high toxicity. This study assessed atezolizumab (anti-programmed death-ligand 1 [PD-L1]) as treatment for metastatic urothelial cancer in cisplatin-ineligible patients.
Methods: For this single-arm, multicentre, phase 2 study, in 47 academic medical centres and community oncology practices in seven countries in North America and Europe, we recruited previously untreated patients with locally advanced or metastatic urothelial cancer who were cisplatin ineligible. Patients were given 1200 mg intravenous atezolizumab every 21 days until progression. The primary endpoint was independently confirmed objective response rate per Response Evaluation Criteria in Solid Tumors version 1.1 (central review), assessed in prespecified subgroups based on PD-L1 expression and in all patients. All participants who received one or more doses of atezolizumab were included in the primary and safety analyses. This study was registered with ClinicalTrials.gov, number NCT02108652.
Findings: Between June 9, 2014, and March 30, 2015, we enrolled 123 patients, of whom 119 received one or more doses of atezolizumab. At 17·2 months' median follow-up, the objective response rate was 23% (95% CI 16 to 31), the complete response rate was 9% (n=11), and 19 of 27 responses were ongoing. Median response duration was not reached. Responses occurred across all PD-L1 and poor prognostic factor subgroups. Median progression-free survival was 2·7 months (2·1 to 4·2). Median overall survival was 15·9 months (10·4 to not estimable). Tumour mutation load was associated with response. Treatment-related adverse events that occurred in 10% or more of patients were fatigue (36 [30%] patients), diarrhoea (14 [12%] patients), and pruritus (13 [11%] patients). One treatment-related death (sepsis) occurred. Nine (8%) patients had an adverse event leading to treatment discontinuation. Immune-mediated events occurred in 14 (12%) patients.
Interpretation: Atezolizumab showed encouraging durable response rates, survival, and tolerability, supporting its therapeutic use in untreated metastatic urothelial cancer.
Funding: F Hoffmann-La Roche, Genentech.
Trial registration: ClinicalTrials.gov NCT02302807 NCT02807636 NCT02108652.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures
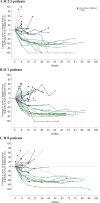

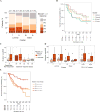
Comment in
-
Immune-checkpoint blockade in cisplatin-ineligible patients with urothelial cancer.Lancet. 2017 Jan 7;389(10064):6-7. doi: 10.1016/S0140-6736(16)32516-8. Epub 2016 Dec 8. Lancet. 2017. PMID: 27939401 No abstract available.
-
Urological cancer: Atezolizumab: an alternative to cisplatin?Nat Rev Clin Oncol. 2017 Mar;14(3):139. doi: 10.1038/nrclinonc.2016.222. Epub 2016 Dec 29. Nat Rev Clin Oncol. 2017. PMID: 28031559 No abstract available.
-
Bladder cancer: Atezolizumab: an alternative to cisplatin?Nat Rev Urol. 2017 Feb;14(2):67. doi: 10.1038/nrurol.2016.271. Epub 2016 Dec 29. Nat Rev Urol. 2017. PMID: 28031561 No abstract available.
References
-
- National cancer institute surveillance, epidemiology, and end results program. SEER cancer statistics factsheets: Bladder cancer. [accessed August 12, 2016]; http://seer.cancer.gov/statfacts/html/urinb.html.
-
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase no. 11. [accessed September 13, 2016]; http://globocan.iarc.fr/Pages/summary_table_site_sel.aspx.
-
- Loehrer PJ, Sr, Einhorn LH, Elson PJ, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: A cooperative group study. J Clin Oncol. 1992;10:1066–73. - PubMed
-
- Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer "unfit" for cisplatin-based chemotherapy. J Clin Oncol. 2011;29:2432–8. - PubMed
-
- Bellmunt J, Orsola A, Leow JJ, et al. Bladder cancer: ESMO practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii40–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

