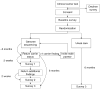
Design of a randomized controlled trial for genomic carrier screening in healthy patients seeking preconception genetic testing
- PMID: 27940182
- PMCID: PMC5274557
- DOI: 10.1016/j.cct.2016.12.007
Design of a randomized controlled trial for genomic carrier screening in healthy patients seeking preconception genetic testing
Abstract
Population-based carrier screening is limited to well-studied or high-impact genetic conditions for which the benefits may outweigh the associated harms and costs. As the cost of genome sequencing declines and availability increases, the balance of risks and benefits may change for a much larger number of genetic conditions, including medically actionable additional findings. We designed an RCT to evaluate genomic clinical sequencing for women and partners considering a pregnancy. All results are placed into the medical record for use by healthcare providers. Through quantitative and qualitative measures, including baseline and post result disclosure surveys, post result disclosure interviews, 1-2year follow-up interviews, and team journaling, we are obtaining data about the clinical and personal utility of genomic carrier screening in this population. Key outcomes include the number of reportable carrier and additional findings, and the comparative cost, utilization, and psychosocial impacts of usual care vs. genomic carrier screening. As the study progresses, we will compare the costs of genome sequencing and usual care as well as the cost of screening, pattern of use of genetic or mental health counseling services, number of outpatient visits, and total healthcare costs. This project includes novel investigation into human reactions and responses from would-be parents who are learning information that could both affect a future pregnancy and their own health.
Keywords: Clinical trial; Genetic testing; Genetics; Genome sequencing; Preconception.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
None of the authors has any conflicts of interests to disclose.
Figures
References
-
- American College of Medical Genetics and American College of Obstetricians and Gynecologists. Preconception and Prenatal Carrier Screening for Cystic Fibrosis: Clinical and Laboratory Guidelines. 2001
-
- American College of Obstetricians and Gynecologists. Update on carrier screening for cystic fibrosis, ACOG. 2011;486:1028–1031. - PubMed
-
- ACOG Committee on genetics. ACOG committee opinion. 318. Screening for Tay-Sachs Disease; Oct, 2005. 2005. - PubMed
-
- The American Congress of Obstetricians and Gynecologists. Spinal Muscular Atrophy. 2009
-
- The American Congress of Obstetricians and Gynecologists. Carrier Screening for Fragile X Syndrome. 2010 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials