Randomized controlled dissemination study of community-to-clinic navigation to promote CRC screening: Study design and implications
- PMID: 27940183
- PMCID: PMC6386159
- DOI: 10.1016/j.cct.2016.12.006
Randomized controlled dissemination study of community-to-clinic navigation to promote CRC screening: Study design and implications
Abstract
Introduction: Regular screening facilitates early diagnosis of colorectal cancer (CRC) and reduction of CRC morbidity and mortality. Screening rates for minorities and low-income populations remain suboptimal. Provider referral for CRC screening is one of the strongest predictors of adherence, but referrals are unlikely among those who have no clinic home (common among poor and minority populations).
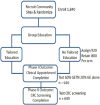
Methods/study design: This group randomized controlled study will test the effectiveness of an evidence based tailored messaging intervention in a community-to-clinic navigation context compared to no navigation. Multicultural, underinsured individuals from community sites will be randomized (by site) to receive CRC screening education only, or education plus navigation. In Phase I, those randomized to education plus navigation will be guided to make a clinic appointment to receive a provider referral for CRC screening. Patients attending clinic appointments will continue to receive navigation until screened (Phase II) regardless of initial arm assignment. We hypothesize that those receiving education plus navigation will be more likely to attend clinic appointments (H1) and show higher rates of screening (H2) compared to those receiving education only. Phase I group assignment will be used as a control variable in analysis of screening follow-through in Phase II. Costs per screening achieved will be evaluated for each condition and the RE-AIM framework will be used to examine dissemination results.
Conclusion: The novelty of our study design is the translational dissemination model that will allow us to assess the real-world application of an efficacious intervention previously tested in a randomized controlled trial.
Copyright © 2016. Published by Elsevier Inc.
Figures
Similar articles
-
Rationale and design of Mi-CARE: The mile square colorectal cancer screening, awareness and referral and education project.Contemp Clin Trials. 2017 Jan;52:75-79. doi: 10.1016/j.cct.2016.11.009. Epub 2016 Nov 22. Contemp Clin Trials. 2017. PMID: 27888090 Free PMC article.
-
Patient Navigation Plus Tailored Digital Video Disc Increases Colorectal Cancer Screening Among Low-Income and Minority Patients Who Did Not Attend a Scheduled Screening Colonoscopy: A Randomized Trial.Ann Behav Med. 2024 Apr 11;58(5):314-327. doi: 10.1093/abm/kaae013. Ann Behav Med. 2024. PMID: 38470961 Free PMC article. Clinical Trial.
-
Comparing the effect of a decision aid plus patient navigation with usual care on colorectal cancer screening completion in vulnerable populations: study protocol for a randomized controlled trial.Trials. 2014 Jul 8;15:275. doi: 10.1186/1745-6215-15-275. Trials. 2014. PMID: 25004983 Free PMC article. Clinical Trial.
-
The Role of Patient Navigation on Colorectal Cancer Screening Completion and Education: a Review of the Literature.J Cancer Educ. 2018 Apr;33(2):251-259. doi: 10.1007/s13187-016-1140-0. J Cancer Educ. 2018. PMID: 27878766 Review.
-
Systematic Review of Interventions to Increase Stool Blood Colorectal Cancer Screening in African Americans.J Community Health. 2021 Feb;46(1):232-244. doi: 10.1007/s10900-020-00867-z. J Community Health. 2021. PMID: 32583358 Free PMC article.
Cited by
-
Adaptation of colorectal cancer screening tailored navigation content for American Indian communities and early results using the intervention.Implement Sci Commun. 2022 Jan 28;3(1):6. doi: 10.1186/s43058-022-00253-x. Implement Sci Commun. 2022. PMID: 35090575 Free PMC article.
-
Startup and implementation costs of a colorectal cancer screening tailored navigation research study.Eval Program Plann. 2021 Apr;85:101907. doi: 10.1016/j.evalprogplan.2021.101907. Epub 2021 Jan 29. Eval Program Plann. 2021. PMID: 33561756 Free PMC article. Clinical Trial.
References
-
- National Cancer Institute (NCI) The colorectal progress review report. http://progressreport.cancer.gov/doc_detail.asp?pid=0&did=0&chid=72&coid... (Accessed February 1 2008)
-
- Richardson LC, Rim SH, Plescia M. Vital signs: Colorectal cancer screening among adults aged 50–75 years United States. Morbidity and Mortality Weekly Report, 2010. 2008;59:1–4. Centers for Disease Control and Prevention Web site. http:www.cdc.gov/mmwr/preview/mmwrhtml/mm5926a3.htm?s_cid=mm5926a3_w. Updated 2009. Accessed August, 31, 2010. - PubMed
-
- Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15(2):389–394. - PubMed
-
- National Colorectal Cancer Roundtable. 80% by 2018 Pledge. http://nccrt.org/tools/80-percent-by-2018/80-percent-by-2018-pledge/ (Accessed October 23, 2016)
-
- National Cancer Institute (NCI) Colorectal cancer screening in primary care practice (PAR-02-042) http://www.grants1.nih.gov/grants/guide/pa-files/PAR-02-042.2002 (Updated 2001. Accessed January 24, 2008)
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical