Cost Utility Analysis of Topical Steroids Compared With Dietary Elimination for Treatment of Eosinophilic Esophagitis
- PMID: 27940272
- PMCID: PMC5440206
- DOI: 10.1016/j.cgh.2016.11.032
Cost Utility Analysis of Topical Steroids Compared With Dietary Elimination for Treatment of Eosinophilic Esophagitis
Abstract
Background & aims: Topical corticosteroids or dietary elimination are recommended as first-line therapies for eosinophilic esophagitis, but data to directly compare these therapies are scant. We performed a cost utility comparison of topical corticosteroids and the 6-food elimination diet (SFED) in treatment of eosinophilic esophagitis, from the payer perspective.
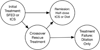
Methods: We used a modified Markov model based on current clinical guidelines, in which transition between states depended on histologic response simulated at the individual cohort-member level. Simulation parameters were defined by systematic review and meta-analysis to determine the base-case estimates and bounds of uncertainty for sensitivity analysis. Meta-regression models included adjustment for differences in study and cohort characteristics.
Results: In the base-case scenario, topical fluticasone was about as effective as SFED but more expensive at a 5-year time horizon ($9261.58 vs $5719.72 per person). SFED was more effective and less expensive than topical fluticasone and topical budesonide in the base-case scenario. Probabilistic sensitivity analysis revealed little uncertainty in relative treatment effectiveness. There was somewhat greater uncertainty in the relative cost of treatments; most simulations found SFED to be less expensive.
Conclusions: In a cost utility analysis comparing topical corticosteroids and SFED for first-line treatment of eosinophilic esophagitis, the therapies were similar in effectiveness. SFED was on average less expensive, and more cost effective in most simulations, than topical budesonide and topical fluticasone, from a payer perspective and not accounting for patient-level costs or quality of life.
Keywords: Cost Effectiveness; Cost Utility; Diet; Eosinophilic Esophagitis; Steroids; Treatment.
Copyright © 2017 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE) Am J Gastroenterol. 2013;108:679–692. quiz 693. - PubMed
-
- Aceves SS, Bastian JF, Newbury RO, et al. Oral viscous budesonide: a potential new therapy for eosinophilic esophagitis in children. Am J Gastroenterol. 2007;102:2271–2279. quiz 2280. - PubMed
-
- Dohil R, Newbury R, Fox L, et al. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology. 2010;139:418–429. - PubMed
-
- Gupta SK, Vitanza JM, Collins MH. Efficacy and safety of oral budesonide suspension in pediatric patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2015;13:66 e3–76 e3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

