Perioperative management of the bleeding patient
- PMID: 27940453
- PMCID: PMC5155545
- DOI: 10.1093/bja/aew358
Perioperative management of the bleeding patient
Abstract
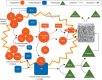
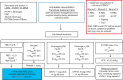
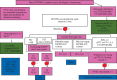
Perioperative bleeding remains a major complication during and after surgery, resulting in increased morbidity and mortality. The principal causes of non-vascular sources of haemostatic perioperative bleeding are a preexisting undetected bleeding disorder, the nature of the operation itself, or acquired coagulation abnormalities secondary to haemorrhage, haemodilution, or haemostatic factor consumption. In the bleeding patient, standard therapeutic approaches include allogeneic blood product administration, concomitant pharmacologic agents, and increasing application of purified and recombinant haemostatic factors. Multiple haemostatic changes occur perioperatively after trauma and complex surgical procedures including cardiac surgery and liver transplantation. Novel strategies for both prophylaxis and therapy of perioperative bleeding include tranexamic acid, desmopressin, fibrinogen and prothrombin complex concentrates. Point-of-care patient testing using thromboelastography, rotational thromboelastometry, and platelet function assays has allowed for more detailed assessment of specific targeted therapy for haemostasis. Strategic multimodal management is needed to improve management, reduce allogeneic blood product administration, and minimize associated risks related to transfusion.
Keywords: coagulopathy; direct oral anticoagulants (DOACs); hemostasis & thrombosis; point-of-care testing; thromboembolism; transfusion algorithm.
© The Author 2016. Published by Oxford University Press on behalf of the British Journal of Anaesthesia. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Sniecinski RM, Chandler WL. Activation of the hemostatic system during cardiopulmonary bypass. Anesth Analg 2011; 113: 1319–33 - PubMed
-
- Despotis GJ, Avidan MS, Hogue CW., Jr., Mechanisms and attenuation of hemostatic activation during extracorporeal circulation. Ann Thorac Surg 2001; 72: S1821–31 - PubMed
-
- Rodeghiero F, Tosetto A, Abshire T, et al. ISTH/SSC bleeding assessment tool: a standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J Thromb Haemost 2010; 8: 2063–5 - PubMed
-
- Achneck HE, Sileshi B, Jamiolkowski RM, Albala DM, Shapiro ML, Lawson JH. A comprehensive review of topical hemostatic agents: efficacy and recommendations for use. Ann Surg 2010; 251: 217–28 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

