A general, modular method for the catalytic asymmetric synthesis of alkylboronate esters
- PMID: 27940868
- PMCID: PMC5619240
- DOI: 10.1126/science.aai8611
A general, modular method for the catalytic asymmetric synthesis of alkylboronate esters
Abstract
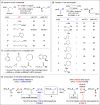
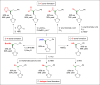
Alkylboron compounds are an important family of target molecules, serving as useful intermediates, as well as end points, in fields such as pharmaceutical science and organic chemistry. Facile transformation of carbon-boron bonds into a wide variety of carbon-X bonds (where X is, for example, carbon, nitrogen, oxygen, or a halogen), with stereochemical fidelity, renders the generation of enantioenriched alkylboronate esters a powerful tool in synthesis. Here we report the use of a chiral nickel catalyst to achieve stereoconvergent alkyl-alkyl couplings of readily available racemic α-haloboronates with organozinc reagents under mild conditions. We demonstrate that this method provides straightforward access to a diverse array of enantioenriched alkylboronate esters, in which boron is bound to a stereogenic carbon, and we highlight the utility of these compounds in synthesis.
Copyright © 2016, American Association for the Advancement of Science.
Figures


References
-
- Hall DG, editor. Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials. 1 and 2 Wiley–VCH; 2011.
-
- Miyaura N, Yamamoto Y. In: Comprehensive Organometallic Chemistry III. Crabtree RH, Mingos DMP, editors. Vol. 9. Elsevier; 2007. Chapter 5.
-
- Brown HC. Hydroboration. Benjamin/Cummings; 1980.
-
- Suzuki A. Angew. Chem. Int. Ed. 2011;50:6722–6737. - PubMed
-
- Smoum R, Rubinstein A, Dembitsky VM, Srebnik M. Chem. Rev. 2012;112:4156–4220. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

