Endosomal Phosphatidylinositol 3-Phosphate Promotes Gephyrin Clustering and GABAergic Neurotransmission at Inhibitory Postsynapses
- PMID: 27941024
- PMCID: PMC5270463
- DOI: 10.1074/jbc.M116.771592
Endosomal Phosphatidylinositol 3-Phosphate Promotes Gephyrin Clustering and GABAergic Neurotransmission at Inhibitory Postsynapses
Abstract
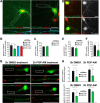
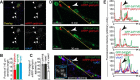
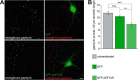
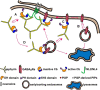
The formation of neuronal synapses and the dynamic regulation of their efficacy depend on the proper assembly of the postsynaptic neurotransmitter receptor apparatus. Receptor recruitment to inhibitory GABAergic postsynapses requires the scaffold protein gephyrin and the guanine nucleotide exchange factor collybistin (Cb). In vitro, the pleckstrin homology domain of Cb binds phosphoinositides, specifically phosphatidylinositol 3-phosphate (PI3P). However, whether PI3P is required for inhibitory postsynapse formation is currently unknown. Here, we investigated the role of PI3P at developing GABAergic postsynapses by using a membrane-permeant PI3P derivative, time-lapse confocal imaging, electrophysiology, as well as knockdown and overexpression of PI3P-metabolizing enzymes. Our results provide the first in cellula evidence that PI3P located at early/sorting endosomes regulates the postsynaptic clustering of gephyrin and GABAA receptors and the strength of inhibitory, but not excitatory, postsynapses in cultured hippocampal neurons. In human embryonic kidney 293 cells, stimulation of gephyrin cluster formation by PI3P depends on Cb. We therefore conclude that the endosomal pool of PI3P, generated by the class III phosphatidylinositol 3-kinase, is important for the Cb-mediated recruitment of gephyrin and GABAA receptors to developing inhibitory postsynapses and thus the formation of postsynaptic membrane specializations.
Keywords: GABA receptor; inositol phospholipid; phosphatidylinositol phosphatase; synapse; vesicles.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures









References
-
- Raiborg C., Schink K. O., and Stenmark H. (2013) Class III phosphatidylinositol 3-kinase and its catalytic product PtdIns3P in regulation of endocytic membrane traffic. FEBS J. 280, 2730–2742 - PubMed
-
- Roth M. G. (2004) Phosphoinositides in constitutive membrane traffic. Physiol. Rev. 84, 699–730 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

