Pre-referral Rectal Artesunate Treatment by Community-Based Treatment Providers in Ghana, Guinea-Bissau, Tanzania, and Uganda (Study 18): A Cluster-Randomized Trial
- PMID: 27941110
- PMCID: PMC5146703
- DOI: 10.1093/cid/ciw631
Pre-referral Rectal Artesunate Treatment by Community-Based Treatment Providers in Ghana, Guinea-Bissau, Tanzania, and Uganda (Study 18): A Cluster-Randomized Trial
Erratum in
-
Erratum.Clin Infect Dis. 2017 May 15;64(10):1469. doi: 10.1093/cid/ciw874. Clin Infect Dis. 2017. PMID: 28329115 Free PMC article. No abstract available.
Abstract
Background: If malaria patients who cannot be treated orally are several hours from facilities for injections, rectal artesunate prior to hospital referral can prevent death and disability. The goal is to reduce death from malaria by having rectal artesunate treatment available and used. How best to do this remains unknown.
Methods: Villages remote from a health facility were randomized to different community-based treatment providers trained to provide rectal artesunate in Ghana, Guinea-Bissau, Tanzania, and Uganda. Prereferral rectal artesunate treatment was provided in 272 villages: 109 through community-based health workers (CHWs), 112 via trained mothers (MUMs), 25 via trained traditional healers (THs), and 26 through trained community-chosen personnel (COMs); episodes eligible for rectal artesunate were established through regular household surveys of febrile illnesses recording symptoms eligible for prereferral treatment. Differences in treatment coverage with rectal artesunate in children aged <5 years in MUM vs CHW (standard-of-care) villages were assessed using the odds ratio (OR); the predictive probability of treatment was derived from a logistic regression analysis, adjusting for heterogeneity between clusters (villages) using random effects.
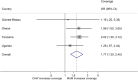
Results: Over 19 months, 54 013 children had 102 504 febrile episodes, of which 32% (31 817 episodes) had symptoms eligible for prereferral therapy; 14% (4460) children received treatment. Episodes with altered consciousness, coma, or convulsions constituted 36.6% of all episodes in treated children. The overall OR of treatment between MUM vs CHW villages, adjusting for country, was 1.84 (95% confidence interval [CI], 1.20-2.83; P = .005). Adjusting for heterogeneity, this translated into a 1.67 higher average probability of a child being treated in MUM vs CHW villages. Referral compliance was 81% and significantly higher with CHWs vs MUMs: 87% vs 82% (risk ratio [RR], 1.1 [95% CI, 1.0-1.1]; P < .0001). There were more deaths in the TH cluster than elsewhere (RR, 2.7 [95% CI, 1.4-5.6]; P = .0040).
Conclusions: Prereferral episodes were almost one-third of all febrile episodes. More than one-third of patients treated had convulsions, altered consciousness, or coma. Mothers were effective in treating patients, and achieved higher coverage than other providers. Treatment access was low.
Clinical trials registration: ISRCTN58046240.
Keywords: Africa; CHWs; coverage; mothers; rectal artesunate.
© 2016 World Health Organization; licensee Oxford Journals.
Figures


References
-
- Dondorp A, Nosten F, Stepniewska N, Day NP, White NJ. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 2005; 366:717–25. - PubMed
-
- Bahl R, Qazi S, Darmstadt GL, Martines J. Why is continuum of care from home to health facilities essential to improve perinatal survival? Semin Perinatol 2010; 34:477–85. - PubMed
-
- World Health Organization. WHO guidelines for the treatment of malaria. 3rd ed Geneva, Switzerland: WHO, 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

