Targeting type I interferon-mediated activation restores immune function in chronic HIV infection
- PMID: 27941243
- PMCID: PMC5199686
- DOI: 10.1172/JCI89488
Targeting type I interferon-mediated activation restores immune function in chronic HIV infection
Abstract
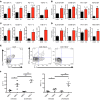
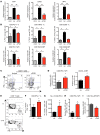
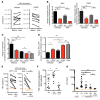
Chronic immune activation, immunosuppression, and T cell exhaustion are hallmarks of HIV infection, yet the mechanisms driving these processes are unclear. Chronic activation can be a driving force in immune exhaustion, and type I interferons (IFN-I) are emerging as critical components underlying ongoing activation in HIV infection. Here, we have tested the effect of blocking IFN-I signaling on T cell responses and virus replication in a murine model of chronic HIV infection. Using HIV-infected humanized mice, we demonstrated that in vivo blockade of IFN-I signaling during chronic HIV infection diminished HIV-driven immune activation, decreased T cell exhaustion marker expression, restored HIV-specific CD8 T cell function, and led to decreased viral replication. Antiretroviral therapy (ART) in combination with IFN-I blockade accelerated viral suppression, further decreased viral loads, and reduced the persistently infected HIV reservoir compared with ART treatment alone. Our data suggest that blocking IFN-I signaling in conjunction with ART treatment can restore immune function and may reduce viral reservoirs during chronic HIV infection, providing validation for IFN-I blockade as a potential therapy for HIV infection.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures

Comment in
-
Interfering with HIV therapy.Sci Transl Med. 2017 Jan 4;9(371):eaal4987. doi: 10.1126/scitranslmed.aal4987. Sci Transl Med. 2017. PMID: 28053154 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

