Prolonged red cell storage before transfusion increases extravascular hemolysis
- PMID: 27941245
- PMCID: PMC5199711
- DOI: 10.1172/JCI90837
Prolonged red cell storage before transfusion increases extravascular hemolysis
Abstract
Background: Some countries have limited the maximum allowable storage duration for red cells to 5 weeks before transfusion. In the US, red blood cells can be stored for up to 6 weeks, but randomized trials have not assessed the effects of this final week of storage on clinical outcomes.
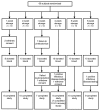
Methods: Sixty healthy adult volunteers were randomized to a single standard, autologous, leukoreduced, packed red cell transfusion after 1, 2, 3, 4, 5, or 6 weeks of storage (n = 10 per group). 51-Chromium posttransfusion red cell recovery studies were performed and laboratory parameters measured before and at defined times after transfusion.
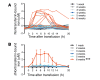
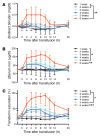
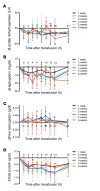
Results: Extravascular hemolysis after transfusion progressively increased with increasing storage time (P < 0.001 for linear trend in the AUC of serum indirect bilirubin and iron levels). Longer storage duration was associated with decreasing posttransfusion red cell recovery (P = 0.002), decreasing elevations in hematocrit (P = 0.02), and increasing serum ferritin (P < 0.0001). After 6 weeks of refrigerated storage, transfusion was followed by increases in AUC for serum iron (P < 0.01), transferrin saturation (P < 0.001), and nontransferrin-bound iron (P < 0.001) as compared with transfusion after 1 to 5 weeks of storage.
Conclusions: After 6 weeks of refrigerated storage, transfusion of autologous red cells to healthy human volunteers increased extravascular hemolysis, saturated serum transferrin, and produced circulating nontransferrin-bound iron. These outcomes, associated with increased risks of harm, provide evidence that the maximal allowable red cell storage duration should be reduced to the minimum sustainable by the blood supply, with 35 days as an attainable goal.REGISTRATION. ClinicalTrials.gov NCT02087514.
Funding: NIH grant HL115557 and UL1 TR000040.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures
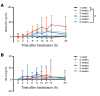
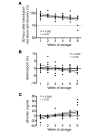
Comment in
- Stored blood: how old is too old? doi: 10.1172/JCI91309
References
-
- Pfuntner A, Wier LM, Stocks C. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb162.pdf Published September 2013. Accessed November 3, 2016.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

