A Systemic Inflammation Mortality Risk Assessment Contingency Table for Severe Sepsis
- PMID: 27941423
- PMCID: PMC5291785
- DOI: 10.1097/PCC.0000000000001029
A Systemic Inflammation Mortality Risk Assessment Contingency Table for Severe Sepsis
Abstract
Objectives: We tested the hypothesis that a C-reactive protein and ferritin-based systemic inflammation contingency table can track mortality risk in pediatric severe sepsis.
Design: Prospective cohort study.
Setting: Tertiary PICU.
Patients: Children with 100 separate admission episodes of severe sepsis were enrolled.
Interventions: Blood samples were attained on day 2 of sepsis and bi-weekly for biomarker batch analysis. A 2 × 2 contingency table using C-reactive protein and ferritin thresholds was developed.
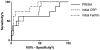
Measurements and main results: A C-reactive protein of 4.08 mg/dL and a ferritin of 1,980 ng/mL were found to be optimal cutoffs for outcome prediction at first sampling (n = 100) using the Youden index. PICU mortality was increased in the "high-risk" C-reactive protein greater than or equal to 4.08 mg/dL and ferritin greater than or equal to 1,980 ng/mL category (6/13 [46.15%]) compared with the "intermediate-risk" C-reactive protein greater than or equal to 4.08 mg/dL and ferritin less than 1,980 ng/mL or C-reactive protein less than 4.08 mg/dL and ferritin greater than or equal to 1,980 ng/mL categories (2/43 [4.65%]), and the "low-risk" C-reactive protein less than 4.08 mg/dL and ferritin less than 1,980 ng/mL category (0/44 [0%]) (odds ratio, 36.43 [95% CI, 6.16-215.21]). The high-risk category was also associated with the development of immunoparalysis (odds ratio, 4.47 [95% CI, 1.34-14.96]) and macrophage activation syndrome (odds ratio, 24.20 [95% CI, 5.50-106.54]). Sixty-three children underwent sequential blood sampling; those who were initially in the low-risk category (n = 24) and those who subsequently migrated (n = 19) to the low-risk category all survived, whereas those who remained in the "at-risk" categories had increased mortality (7/20 [35%]; p < 0.05).
Conclusions: A C-reactive protein- and ferritin-based contingency table effectively assessed mortality risk. Reduction in systemic inflammation below a combined threshold C-reactive protein of 4.08 mg/dL and ferritin of 1,980 ng/mL appeared to be a desired response in children with severe sepsis.
Conflict of interest statement
The authors have no financial relationships relevant to this article to disclose. The authors have no conflicts of interest to disclose.
Figures



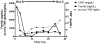
Comment in
-
Ferritin and C-Reactive Protein as Markers of Systemic Inflammation in Sepsis.Pediatr Crit Care Med. 2017 Feb;18(2):194-196. doi: 10.1097/PCC.0000000000001036. Pediatr Crit Care Med. 2017. PMID: 28157797 No abstract available.
References
-
- Watson RS, Carcillo JA, Linde-Zwirble WT, et al. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167:695–701. - PubMed
-
- Odetola FO, Gebremariam A, Freed GL. Patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis. Pediatrics. 2007;119:487–494. - PubMed
-
- McWilliam S, Riordan A. How to use: C-reactive protein. Arch Dis Child Educ Pract Ed. 2010;95:55–58. - PubMed
-
- Schmit X, Vincent JL. The time course of blood C-reactive protein concentrations in relation to the response to initial antimicrobial therapy in patients with sepsis. Infection. 2008;36:213–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 HD050012/HD/NICHD NIH HHS/United States
- UG1 HD049983/HD/NICHD NIH HHS/United States
- UG1 HD050096/HD/NICHD NIH HHS/United States
- U10 HD049981/HD/NICHD NIH HHS/United States
- U10 HD050096/HD/NICHD NIH HHS/United States
- UG1 HD083170/HD/NICHD NIH HHS/United States
- U10 HD049983/HD/NICHD NIH HHS/United States
- U10 HD063106/HD/NICHD NIH HHS/United States
- UG1 HD063108/HD/NICHD NIH HHS/United States
- RL1 HD107773/HD/NICHD NIH HHS/United States
- U10 HD063114/HD/NICHD NIH HHS/United States
- U01 HD049934/HD/NICHD NIH HHS/United States
- UG1 HD049981/HD/NICHD NIH HHS/United States
- R01 GM108618/GM/NIGMS NIH HHS/United States
- U10 HD063108/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

