Role of Circulating MicroRNAs in the Immunopathogenesis of Rejection After Pediatric Lung Transplantation
- PMID: 27941431
- PMCID: PMC5462875
- DOI: 10.1097/TP.0000000000001595
Role of Circulating MicroRNAs in the Immunopathogenesis of Rejection After Pediatric Lung Transplantation
Abstract
Background: Acute rejection (AR) and development of chronic rejection, bronchiolitis obliterans syndrome (BOS) remain major limiting factors for lung transplantation (LTx). This retrospective study is to identify differentially expressed circulating microRNAs (miRNAs) that associate with development of AR and BOS in pediatric lung transplant recipients (LTxR).
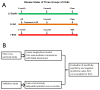
Methods: We determined the circulating levels of 7 selected candidate miRNAs in 14 LTxR with AR, 7 with BOS, and compared them against 13 stable pediatric LTxR at 1, 6, and 12 months after LTx. In addition, 6 AR, 7 BOS, and 8 stable pediatric LTxR, 16 AR, 17 BOS, and 16 stable adult LTxR were included for validation.
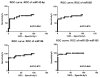
Results: MiR-10a, -195, -133b were significantly lower in AR and miR-144, -142-5p, -155 were higher in AR compared to stable (P < 0.05). In addition, circulating levels of miR-134, -10a, -195, -133b were significantly lower and miR-144, -142-5p, -155 were higher (P < 0.05) with development of BOS. The receiver-operating characteristic demonstrated that miR-142-5p, miR-155, and miR-195 strongly discriminated patients with AR from stable LTxR (P < 0.001 for all comparisons): miR-142-5p (area under the curve [AUC], 0.854), miR-155 (AUC, 0.876), and miR-195 (AUC, 0.872). Further, miR-10a, miR-142-5p, miR-144, and miR-155 strongly discriminated BOS from stable LTxR (P < 0.001 for all comparisons).
Conclusions: We demonstrated that differential expression of circulating miRNAs occurs in LTxR with AR and BOS, suggesting that they can provide not only important clues to pathogenesis but also may serve as potential noninvasive biomarkers for AR and BOS after pediatric LTx.
Figures


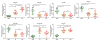
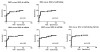
References
-
- Estenne M, Hertz MI. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med. 2002;166(4):440–444. - PubMed
-
- Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21(3):297–310. - PubMed
-
- Peltz M, Edwards LB, Jessen ME, Torres F, Meyer DM. HLA mismatches influence lung transplant recipient survival, bronchiolitis obliterans and rejection: implications for donor lung allocation. J Heart Lung Transplant. 2011;30(4):426–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

